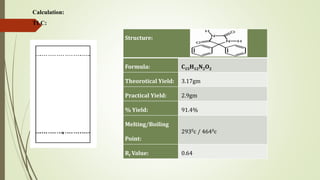
Phenytoin is synthesized from benzil in a multi-step reaction involving a pinacol rearrangement. Benzil and urea are condensed in the presence of base to form an intermediate 1,2-diol, which is then protonated to form an oxonium ion. Dehydration leads to a carbonium ion that undergoes a 1,2 shift of the phenyl ring to form a phenonium ion. Rearrangement of this ion generates the final product, phenytoin, which is isolated by acidification and recrystallization with a 91.4% yield.

![Procedure: [Ref: B. S. Furniss, A. J. Hannaford, P. W. G. Smith, Vogel’s textbook of practical
organic chemistry, 5th edition, Copublished in the United States with John Wiley & Sons, Inc., New
York Page No. 1153
Sachin Kumar Ghosh, Advanced General Organic Chemistry A Modern Approach, Published by
New Central Book Agency (P) Ltd., Kolkata, 2nd edition, Page No. 604-605]
Place 5.3 g (0.0125 mol) of benzil (Expt 6.143), 1.5 g (0.05 mol) of urea, 7.5 ml of 30 per cent
aqueous sodium hydroxide solution and 35 ml of ethanol in a 100- ml round-bottomed flask. Attach
a reflux condenser and boil under reflux using an electric heating mantle for at least 2 hours. Cool
to room temperature, pour the reaction product into 62.5 ml of water and mix thoroughly. Allow to
stand for 15 minutes and then filter under suction to remove an insoluble by-product. Render the
filtrate strongly acidic with concentrated hydrochloric acid, cool in ice-water and immediately filter
off the precipitated product under suction.
Recrystallise at least once from industrial spirit to obtain about 2.8 g (44%) of pure 5,5-
diphenylhydantoin, m.p. 297-298 °C.](https://image.slidesharecdn.com/exp5phenytoin1-210626113119/85/Practical-Experiment-5-Phenytoin-2-320.jpg)
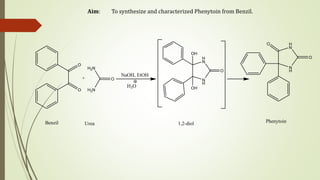
![Principle:
Synthesis of 5,5-diphenylimidazolidine-2,4-dione (1) involves Pinacol-pinacolone
rearrangement reaction. The base catalysed reaction proceeds via an intermediate
heterocyclic pinacol (1,2 diol), which on acidification yields phenytoin.
Step Ι] Condensation of urea and benzil to form glycol (1,2-diol)
Benzil Urea Glycol (1, 2-diol)](https://image.slidesharecdn.com/exp5phenytoin1-210626113119/85/Practical-Experiment-5-Phenytoin-4-320.jpg)
![Step ΙΙ] Protonation of glycol to form oxonium ion
Glycol (1,2-diol) Oxonium ion
Step ΙΙΙ] Dehydration to form corbonium ion.
Carbonium ion](https://image.slidesharecdn.com/exp5phenytoin1-210626113119/85/Practical-Experiment-5-Phenytoin-5-320.jpg)
![Step ΙV] 1,2 shift of phenyl ring to form phenonium ion
Phenonium ion
Step V] Rearrangement of carbonium ion
Rearranged carbonium ion
Step VΙ] Loss of proton to give 5,5-diphenylimidazolidine-2,4-dione (1) (pinacolone)](https://image.slidesharecdn.com/exp5phenytoin1-210626113119/85/Practical-Experiment-5-Phenytoin-6-320.jpg)