The document discusses several key biogeochemical cycles - carbon, oxygen, nitrogen, sulfur, phosphorus - and how they function in ecosystems. It also covers energy flow through ecosystems. Some key points:
1. Biogeochemical cycles circulate essential nutrients through biotic and abiotic components of ecosystems. This maintains the supply and circulation of nutrients that ecosystems depend on.
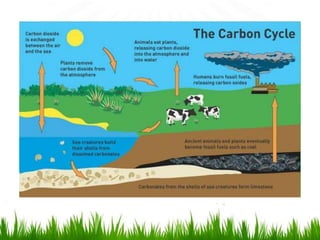
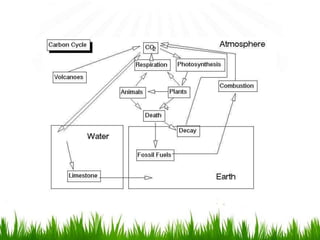
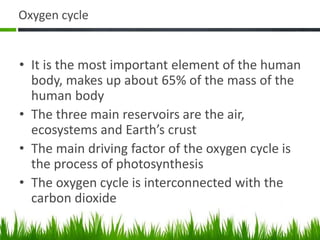
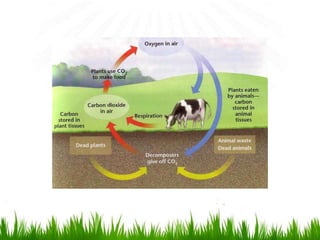
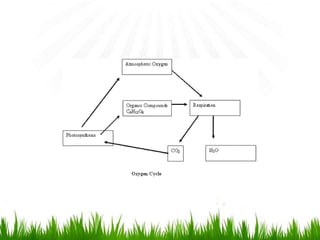
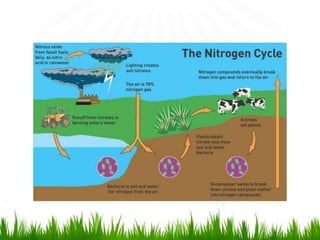
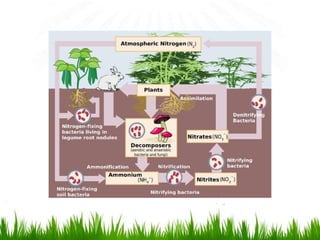
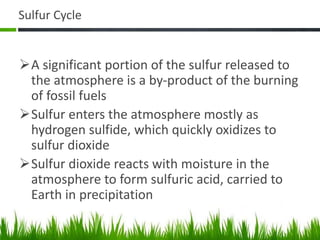
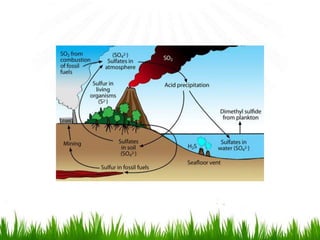
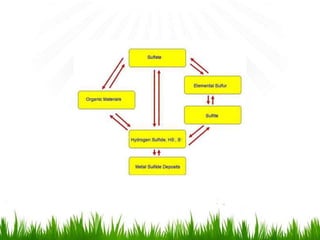
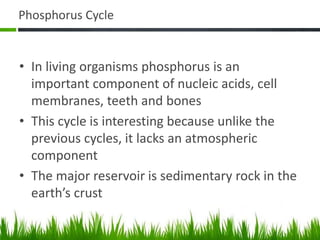
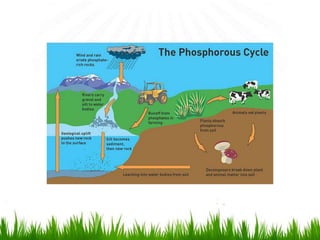
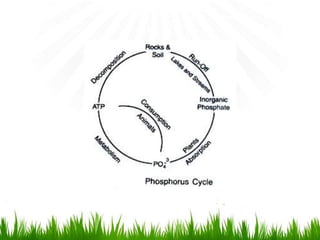
2. Carbon, oxygen and nitrogen cycles are gaseous nutrient cycles, with the atmosphere and oceans as main reservoirs. Sedimentary nutrient cycles include sulfur and phosphorus, with soil, rocks and minerals as primary sources.
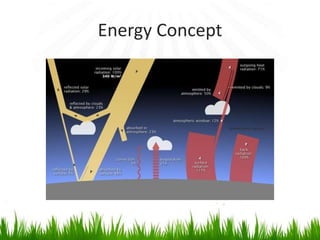
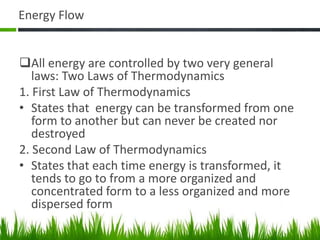
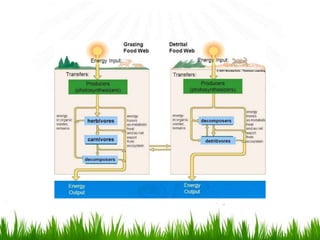
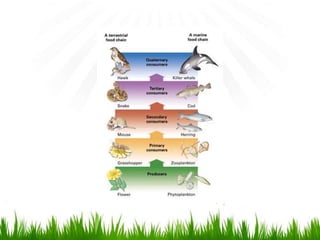
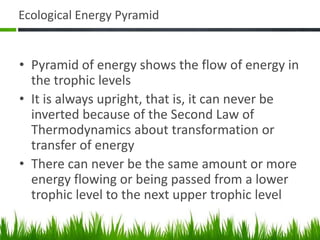
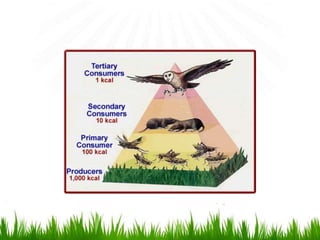
3. Energy from the sun drives biogeochemical cycles and flows through ecosystems via photosynthesis, food chains,