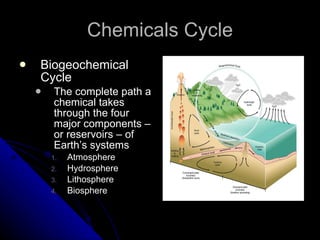
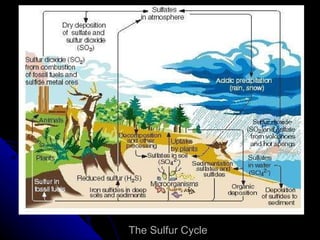
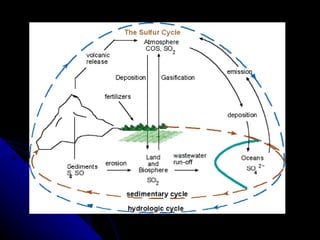
The document summarizes several key biogeochemical cycles, including the carbon, nitrogen, sulfur, phosphorus, hydrologic, rock, and tectonic cycles. It describes the processes involved in each cycle, such as photosynthesis, respiration, nitrogen fixation, and denitrification. It also notes how human activities like burning fossil fuels and agriculture can disrupt these natural cycles.