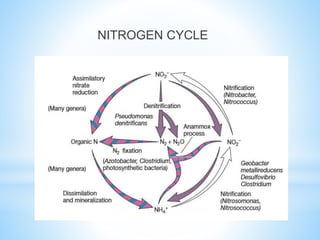
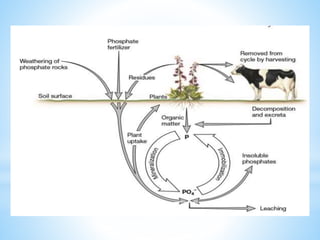
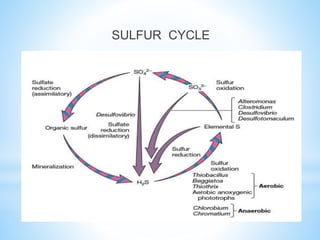
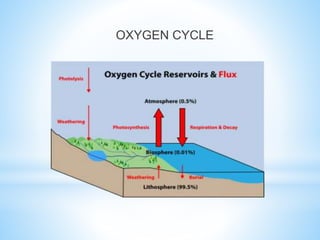
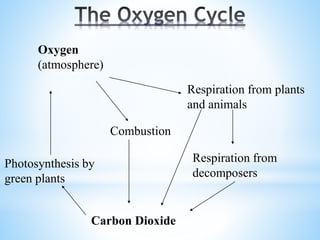
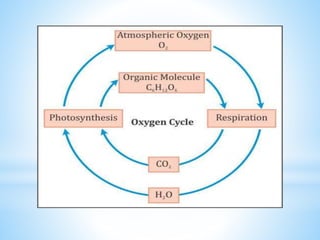
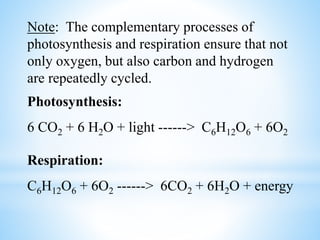
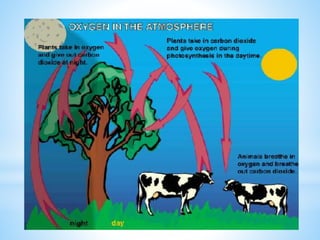
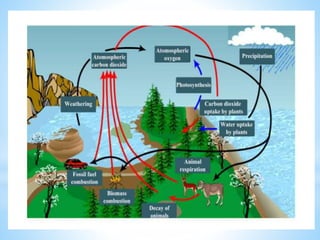
The document summarizes several important biogeochemical cycles, including the carbon, nitrogen, phosphorus, sulfur, and oxygen cycles. It describes how each element is cycled between the biosphere, geosphere, and atmosphere through biological and chemical processes. Microorganisms play a key role in transferring nutrients between different forms and facilitating exchange through oxidation-reduction reactions. All of the cycles are interlinked as they involve the movement of elements between living organisms and their environment.



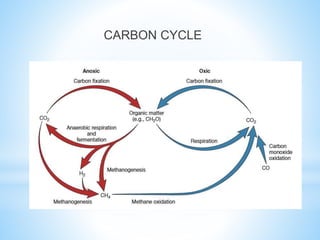
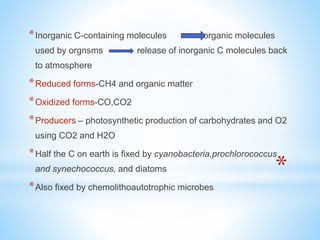
![*Consumers-C containing molecules are incorporated into
body,respiration,release of CO2 and H2O
*Inorganic[CO2] and organic C reduced to CH4 anaerobically,CH4 is
produced by archaea in anoxic habitats
*Decomposers-organic molecules are used as food, it's respiration
release CO2 and H2O
*Most C substrates can be degraded easily with or without O2 except
hydrocarbons and lignin
*Oxic condition-oxidized products such as nitrate, sulfate and CO2
*Anoxic-reduced end products such as ammonium ion ,sulfide and
CH4](https://image.slidesharecdn.com/biogeochemicalcycles-181126131216/85/Biogeochemical-cycles-6-320.jpg)