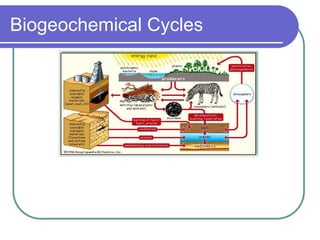
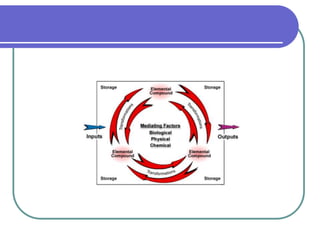
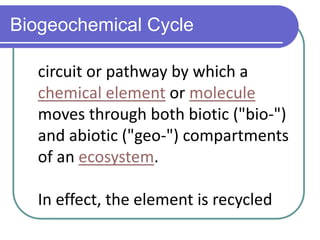
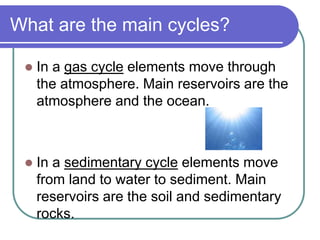
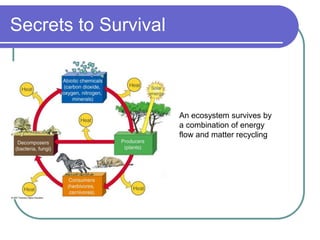
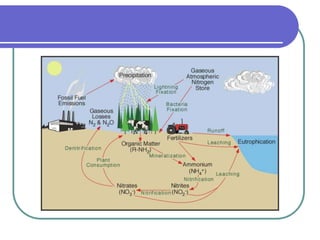
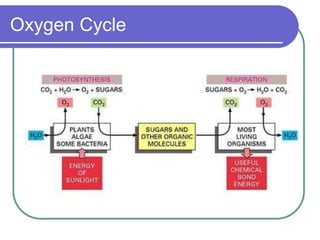
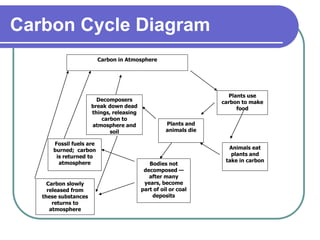
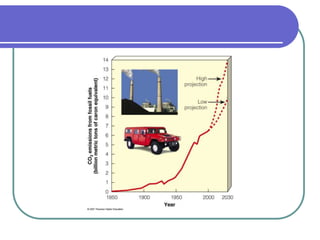
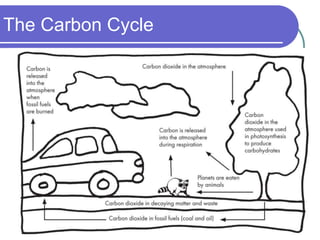
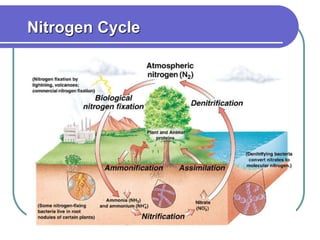
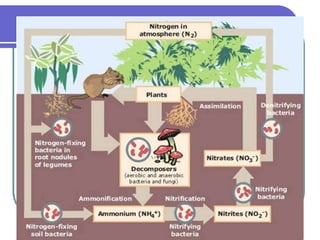
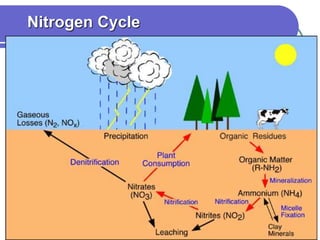
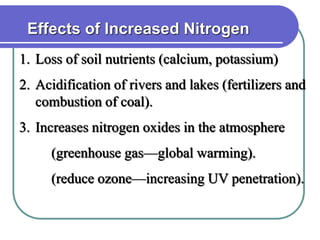
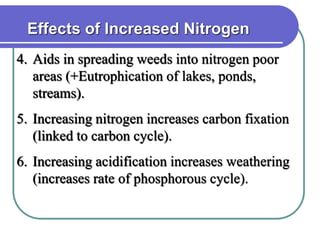
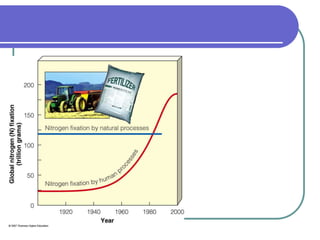
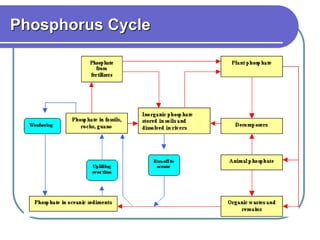
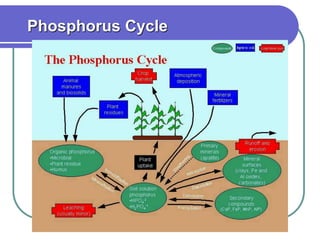
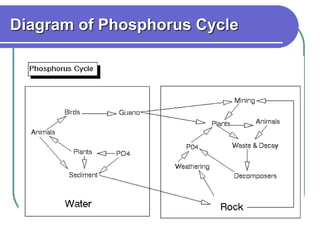
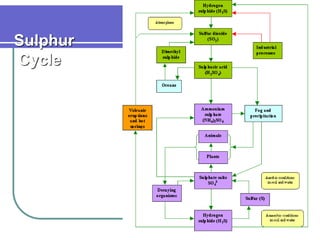
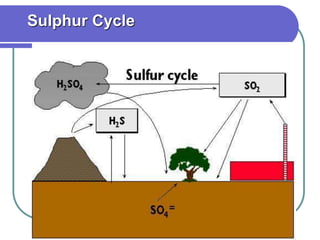
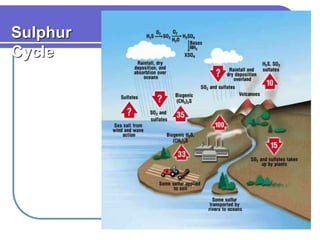
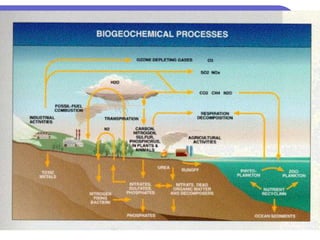
Biogeochemical cycles describe the movement of elements and molecules through biotic and abiotic components of ecosystems. Key cycles include carbon, nitrogen, oxygen, phosphorus, and sulfur. In these cycles, matter is transferred between living organisms, non-living matter like soil and water, and long-term stores like fossil fuels and sedimentary rocks. The recycling of nutrients through biogeochemical cycles is essential for sustaining life on Earth.