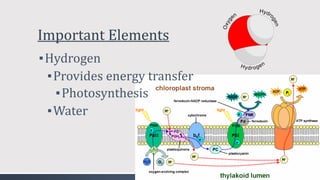
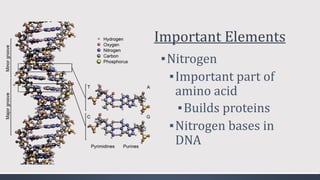
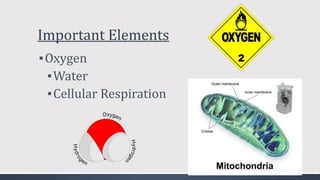
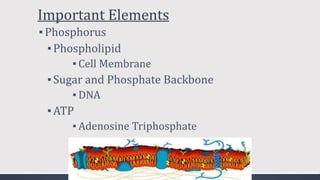
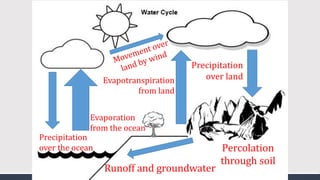
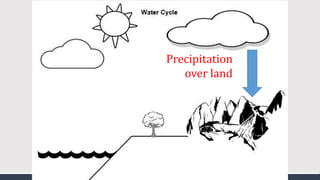
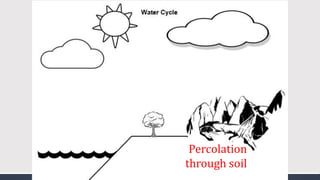
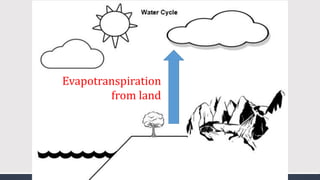
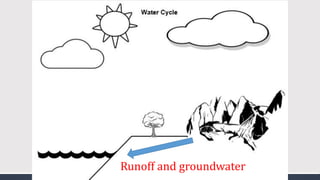
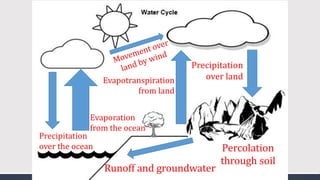
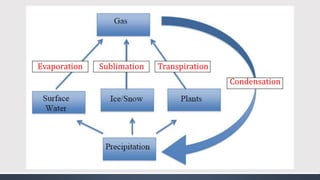
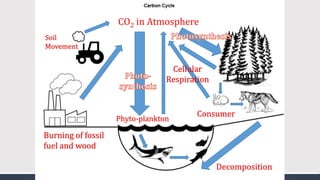
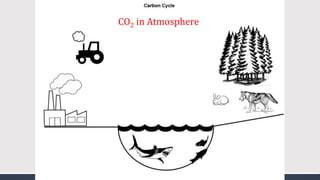
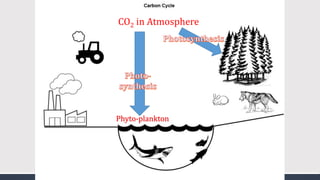
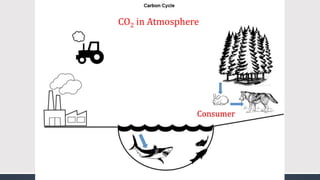
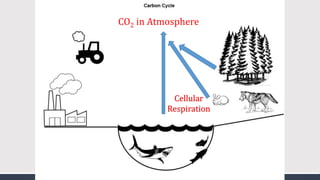
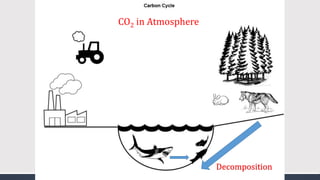
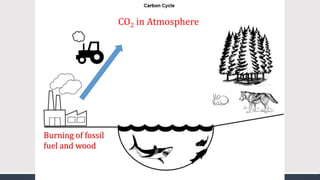
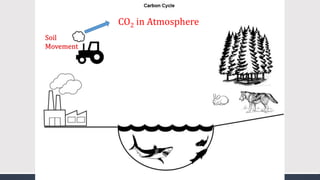
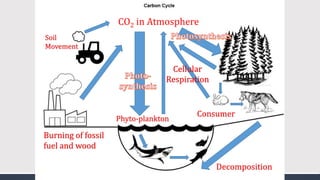
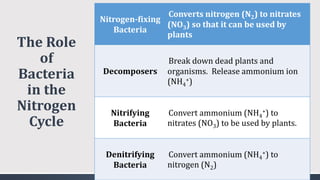
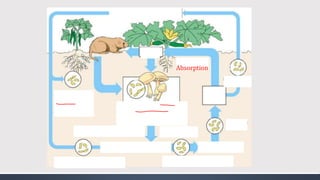
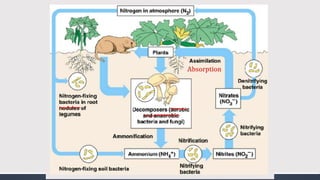
The document discusses several biogeochemical cycles including the water, carbon, and nitrogen cycles. The water cycle involves evaporation, condensation, precipitation, and transpiration moving water between the atmosphere and Earth's surface. The carbon cycle moves carbon between the atmosphere, organisms, oceans, fossil fuels, and soil through photosynthesis, respiration, and decomposition. Nitrogen is converted between gas and organic forms through nitrogen fixation by bacteria, decomposition, and nitrification and denitrification by other bacteria.