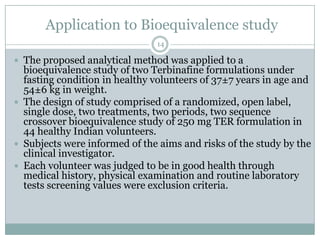
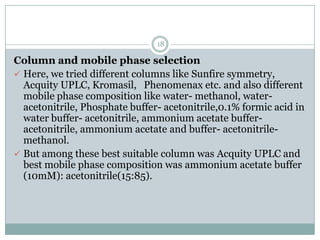
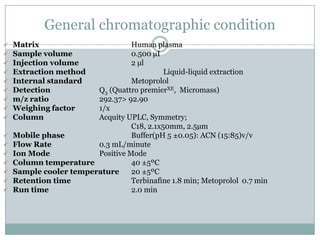
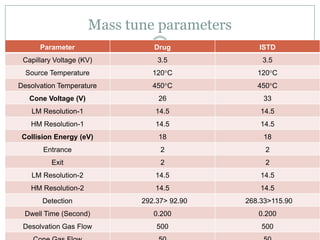
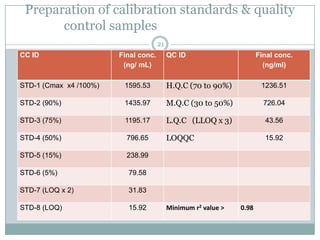
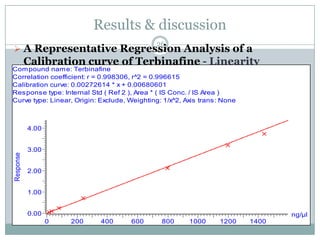
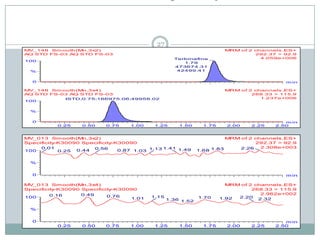
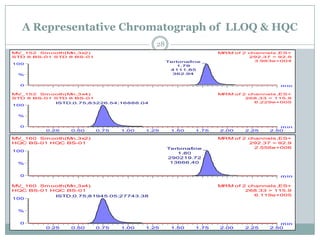
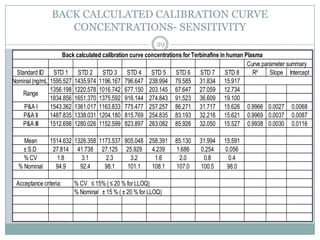
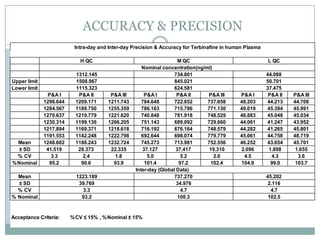
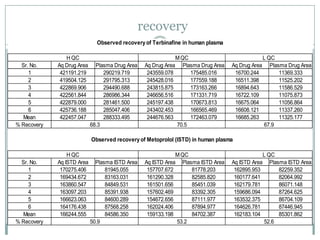
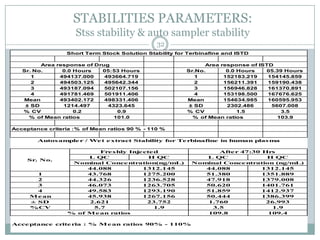
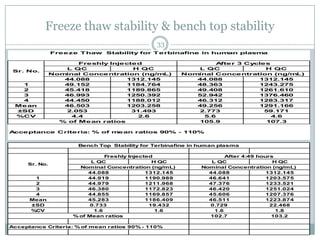
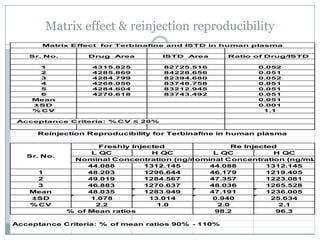
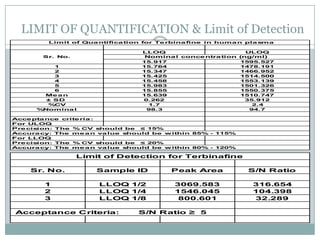
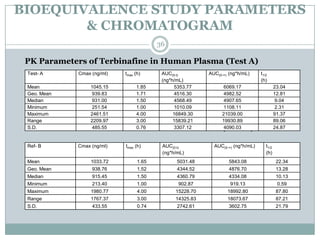
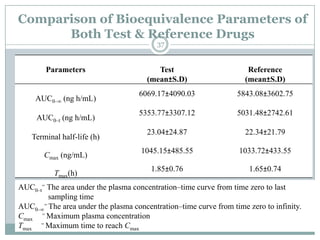
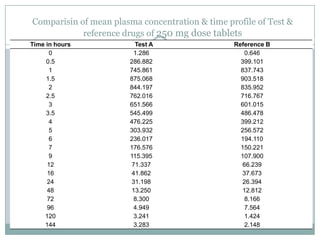
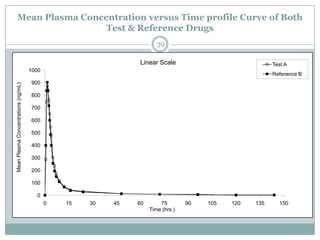
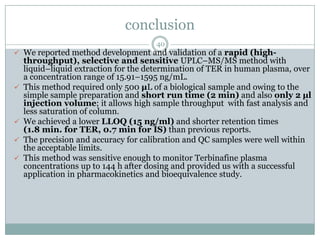
The document describes the development and validation of a UPLC-MS/MS method for quantifying terbinafine in human plasma. The method uses liquid-liquid extraction of terbinafine and an internal standard from plasma followed by separation using UPLC and detection by MS/MS. The method was validated per FDA guidelines and applied to analyze samples from a bioequivalence study comparing two terbinafine formulations in healthy volunteers. Key objectives were to develop a sensitive, specific, high-throughput method to support clinical pharmacokinetic studies of terbinafine.

![Introduction- Drug profile (terbinafine)
7
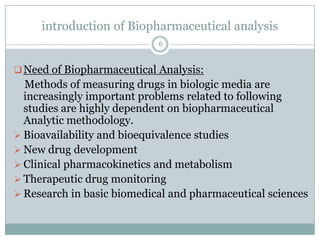
Terbinafine (TER) is [(E)-N-(6, 6-dimethyl-2-heptene-
4-ynyl)-N-methyl-1-naphthalene methanamine]
synthetic allylamine antifungal compound. It is freely
soluble in methanol and methylene chloride, soluble in
ethanol, and slightly soluble in water .
The empirical formula C21H25N with a molecular weight
of 291.43g/mol and the following structural formula:
TERBINAFINE CH3
N C(CH3)3](https://image.slidesharecdn.com/bioequivalence-121105070206-phpapp01/85/Bioequivalence-7-320.jpg)


![Continues…..
13
One of the problems with any AB/BA trial is carry-over, if Carry-
over is present when the effects of the drugs in period 1 are still
noticeable in period 2. For a bioequivalence study this would be the
case if the first plasma level before administration of the drug in
the second period is not 0. If that is the case the washout between the
two periods was not sufficiently long.
The study must have 1] been a single dose study,
2] been in healthy normal volunteers,
3] not been comparing an endogenous
substance,
4] had an adequate washout and
5] used an appropriate
design, analysis and
equivalence must be present.](https://image.slidesharecdn.com/bioequivalence-121105070206-phpapp01/85/Bioequivalence-13-320.jpg)