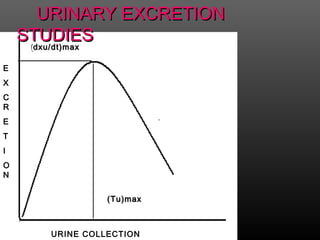
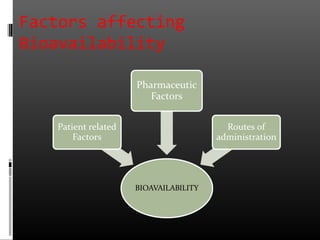
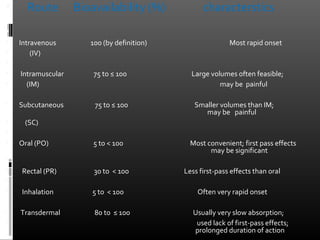
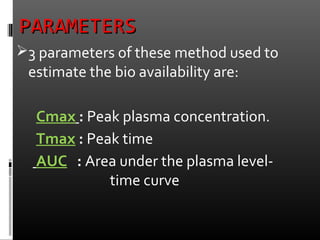
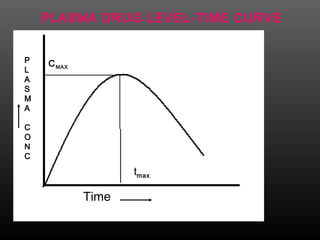
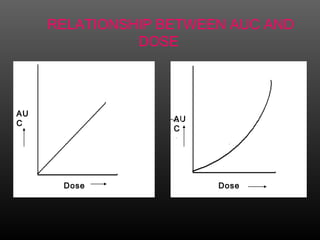
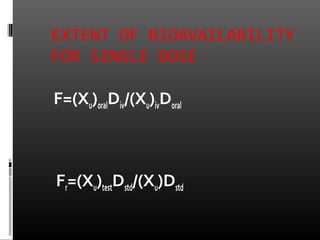This document discusses bioavailability and factors that affect it. It defines bioavailability as the rate and extent to which an administered drug reaches systemic circulation. Factors that can impact bioavailability include pharmaceutical factors like drug properties and dosage form characteristics, patient factors like disease state and gastrointestinal contents, and route of administration. Methods for measuring bioavailability include pharmacokinetic methods using plasma concentration-time profiles or urinary excretion studies, pharmacodynamic methods assessing pharmacological or therapeutic responses, and scintigraphy studies. Approaches to enhance bioavailability involve modifying pharmaceutical, pharmacokinetic, or biological factors.














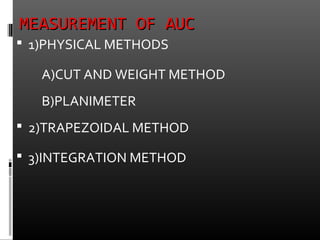

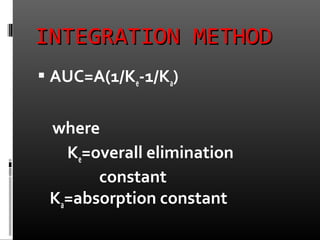
![TRPEZOIDAL METHODTRPEZOIDAL METHOD
It involves the breaking up of the
plasma con vs time profile in to several
trapezoids calculating the area of each
trapezoid and add them to obtain the
AUC
AUC = [(co+c1)(t1-
to)/2]+……… + (cn-1+cn)(tn-tn-1)/2](https://image.slidesharecdn.com/finalppt-130630131212-phpapp01/85/bio-availability-23-320.jpg)
![EXTENT OF BIOAVAILABILITYEXTENT OF BIOAVAILABILITY
FOR SINGLE DOSE STUDYFOR SINGLE DOSE STUDY
F=[AUC]oralDiv/ [AUC]ivDoral
Fr=[AUC]testDstd/[AUC]stdDtest
D = DOSE
ADMINISTERED](https://image.slidesharecdn.com/finalppt-130630131212-phpapp01/85/bio-availability-25-320.jpg)
![EXTENT OF BIOAVAILABILITYEXTENT OF BIOAVAILABILITY
FOR MULTIPLE DOSE STUDYFOR MULTIPLE DOSE STUDY
Fr=[AUC]testDstdTtest/[AUC]stdDtestTstd
T=Dosing interval
Bioavailability can also be measured by peak
plasma concentration at steady state,
Fr=[Cssmax]testDstdTtest/[Cssmax]stdDtestTstd
T=dosing interval](https://image.slidesharecdn.com/finalppt-130630131212-phpapp01/85/bio-availability-26-320.jpg)