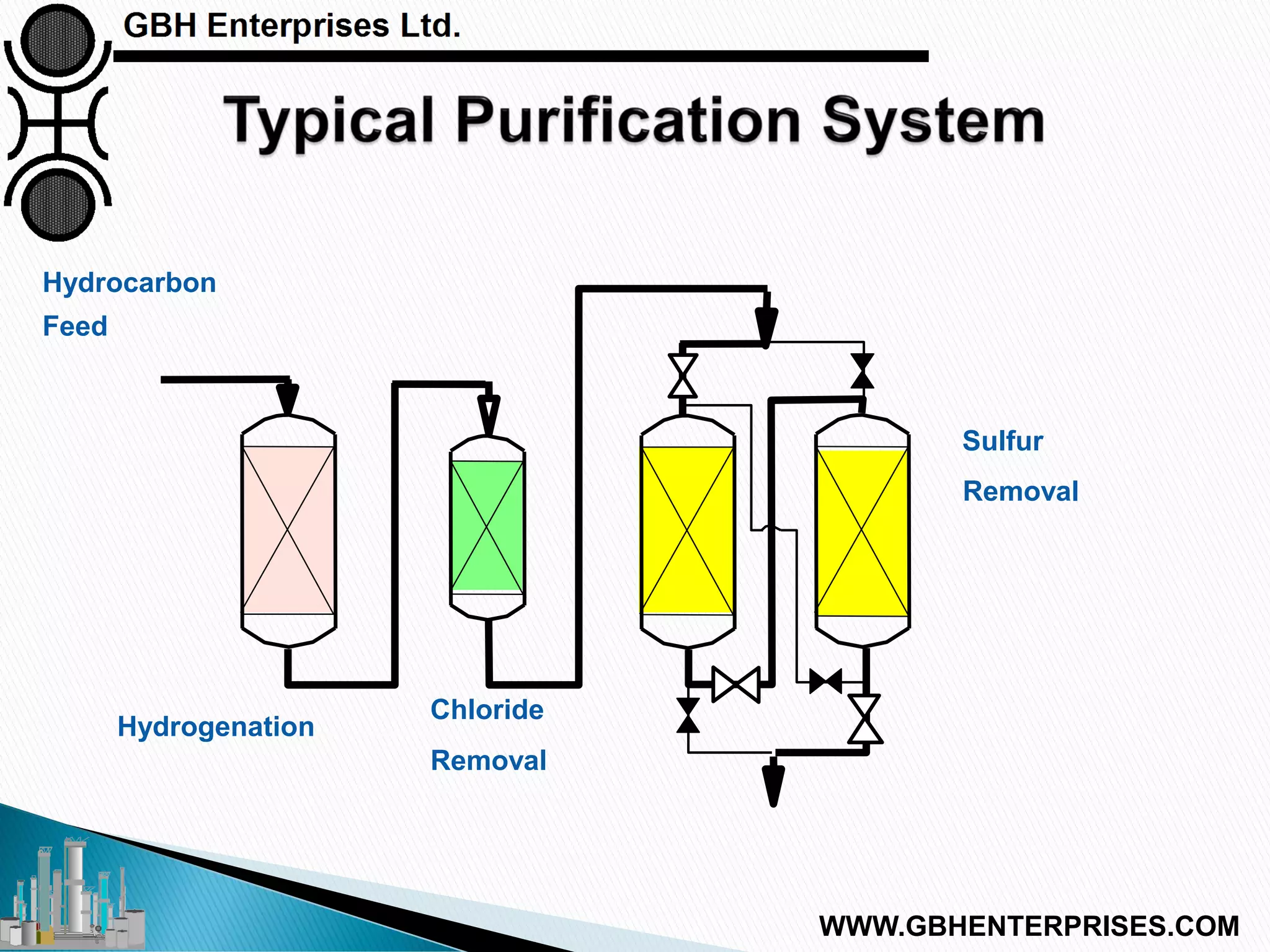
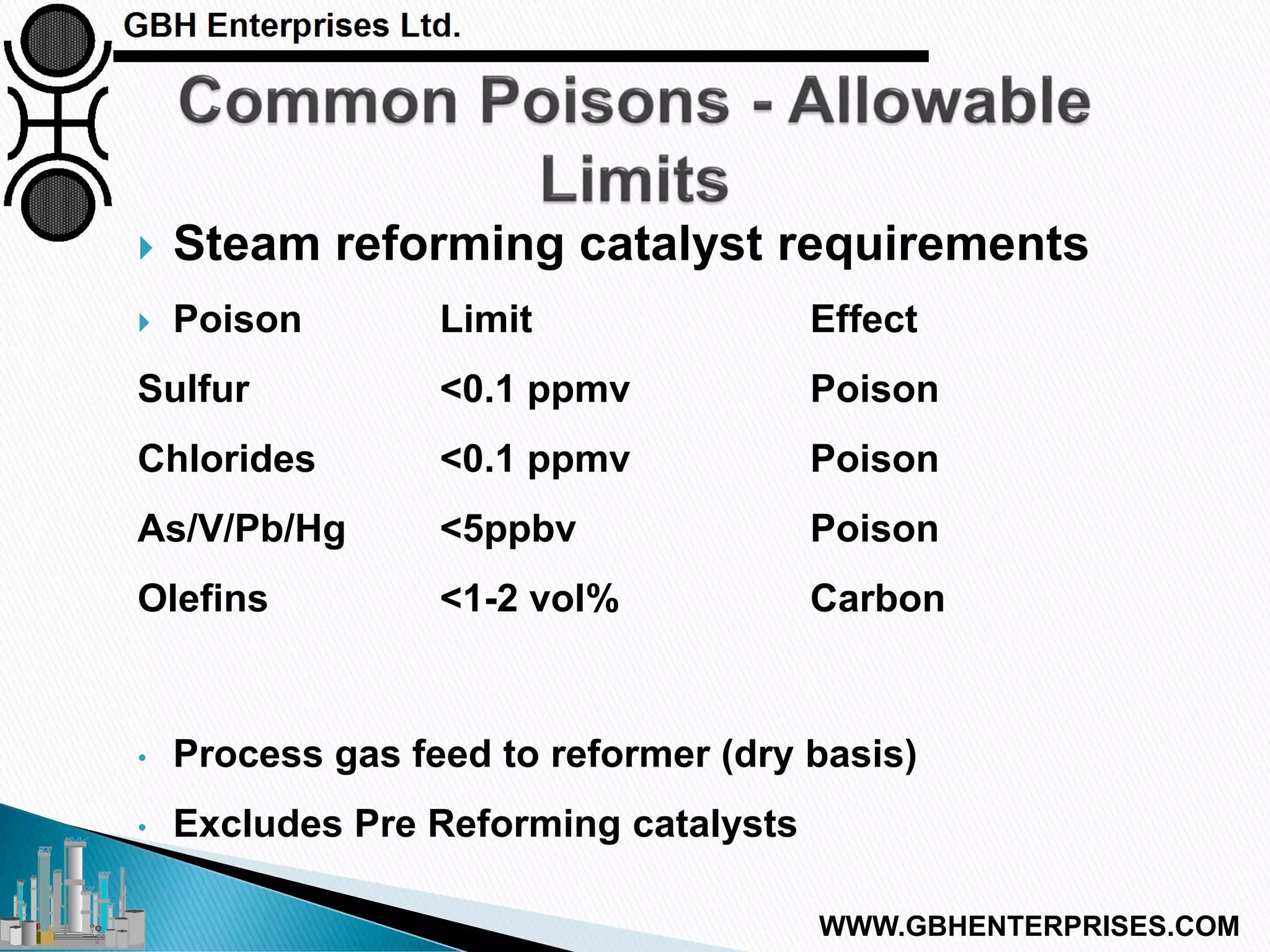
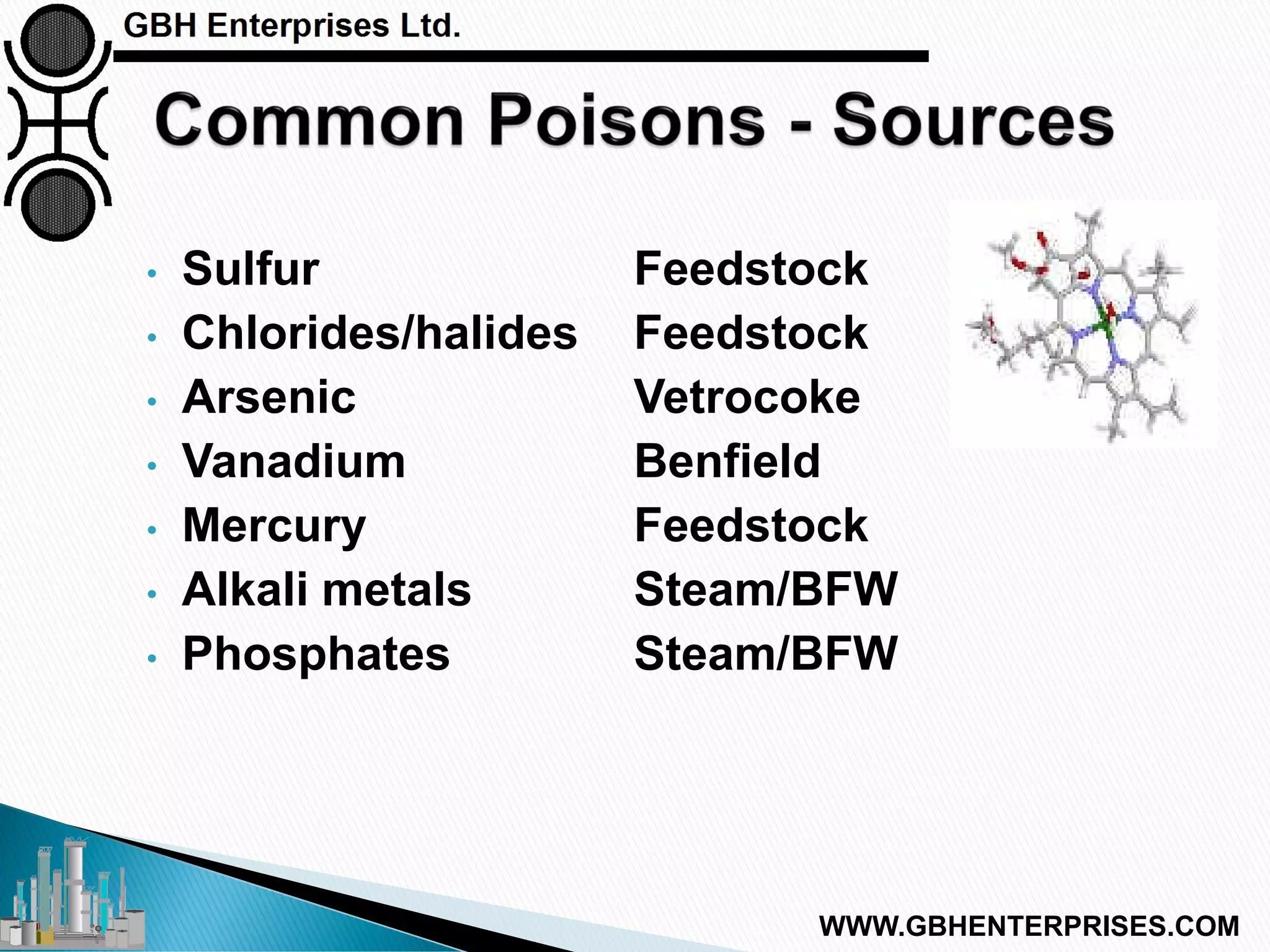
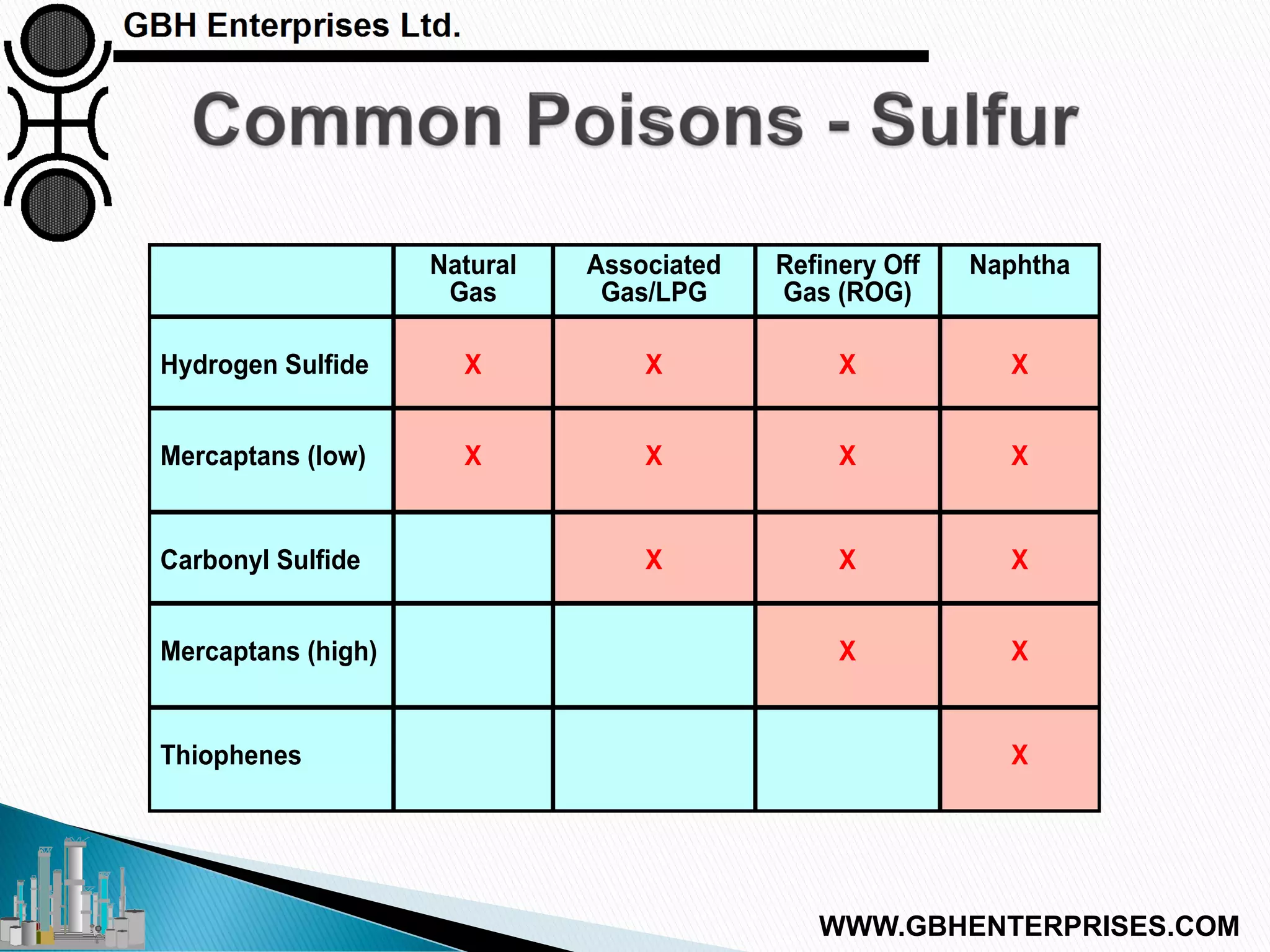
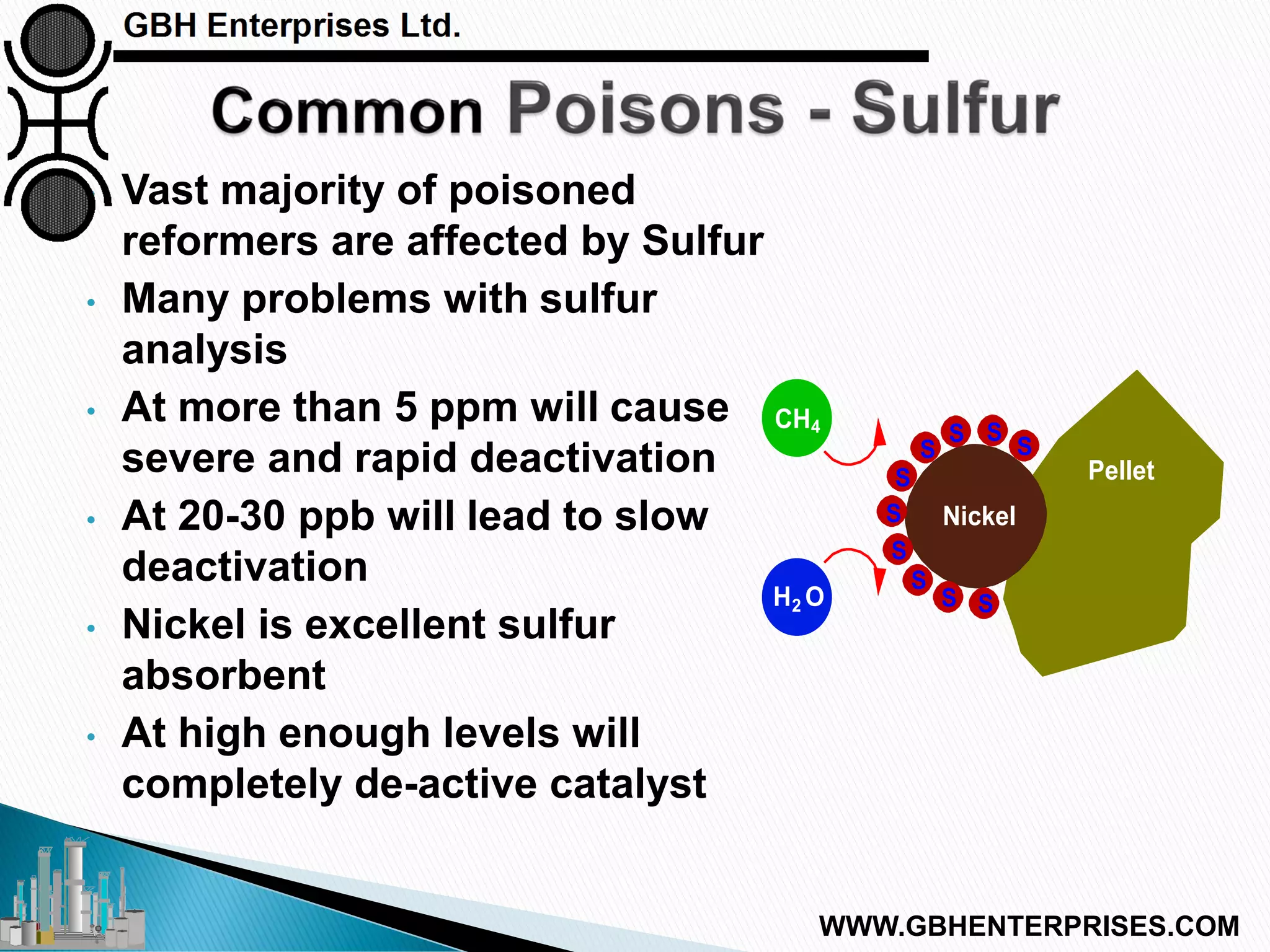
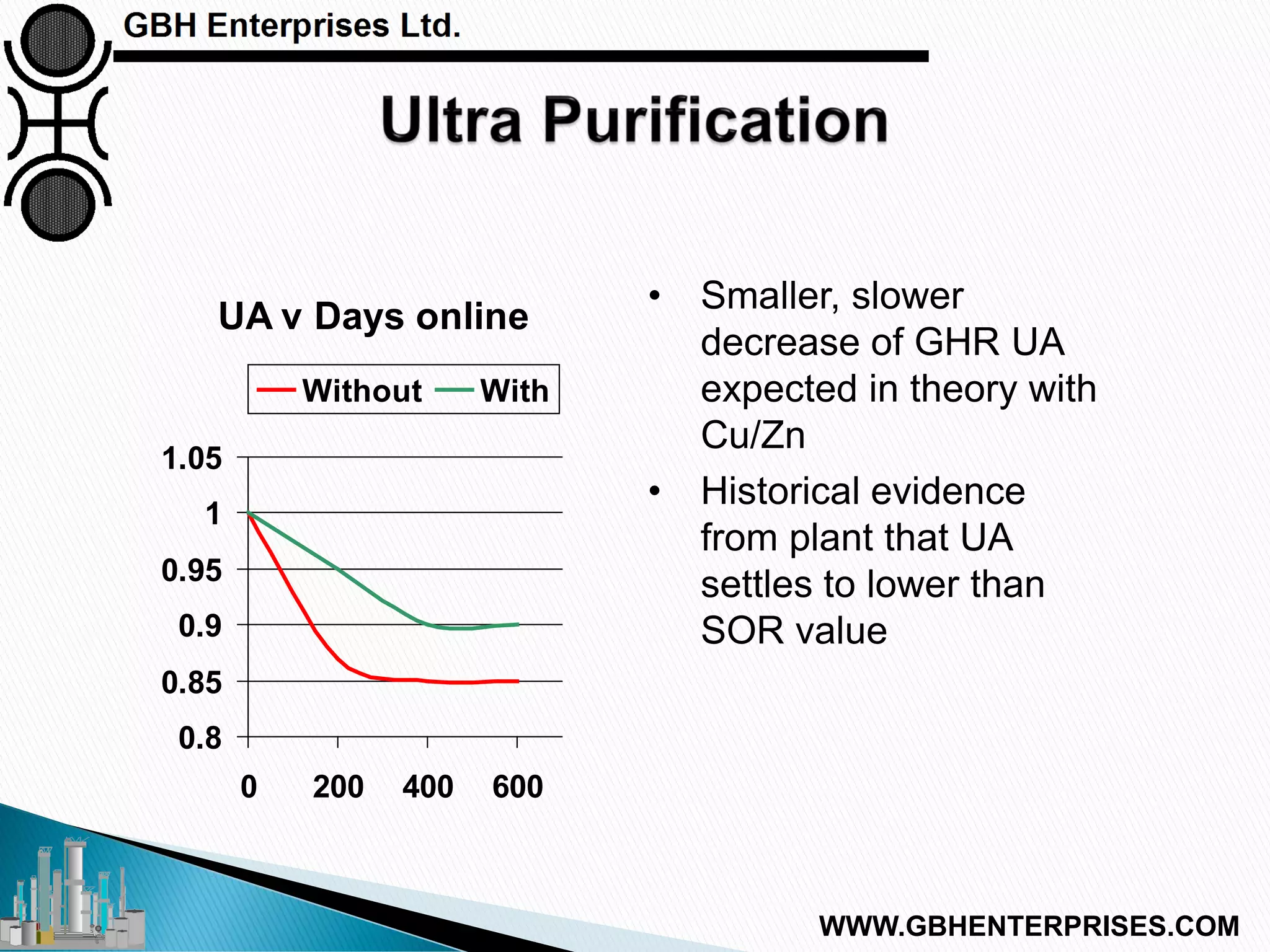
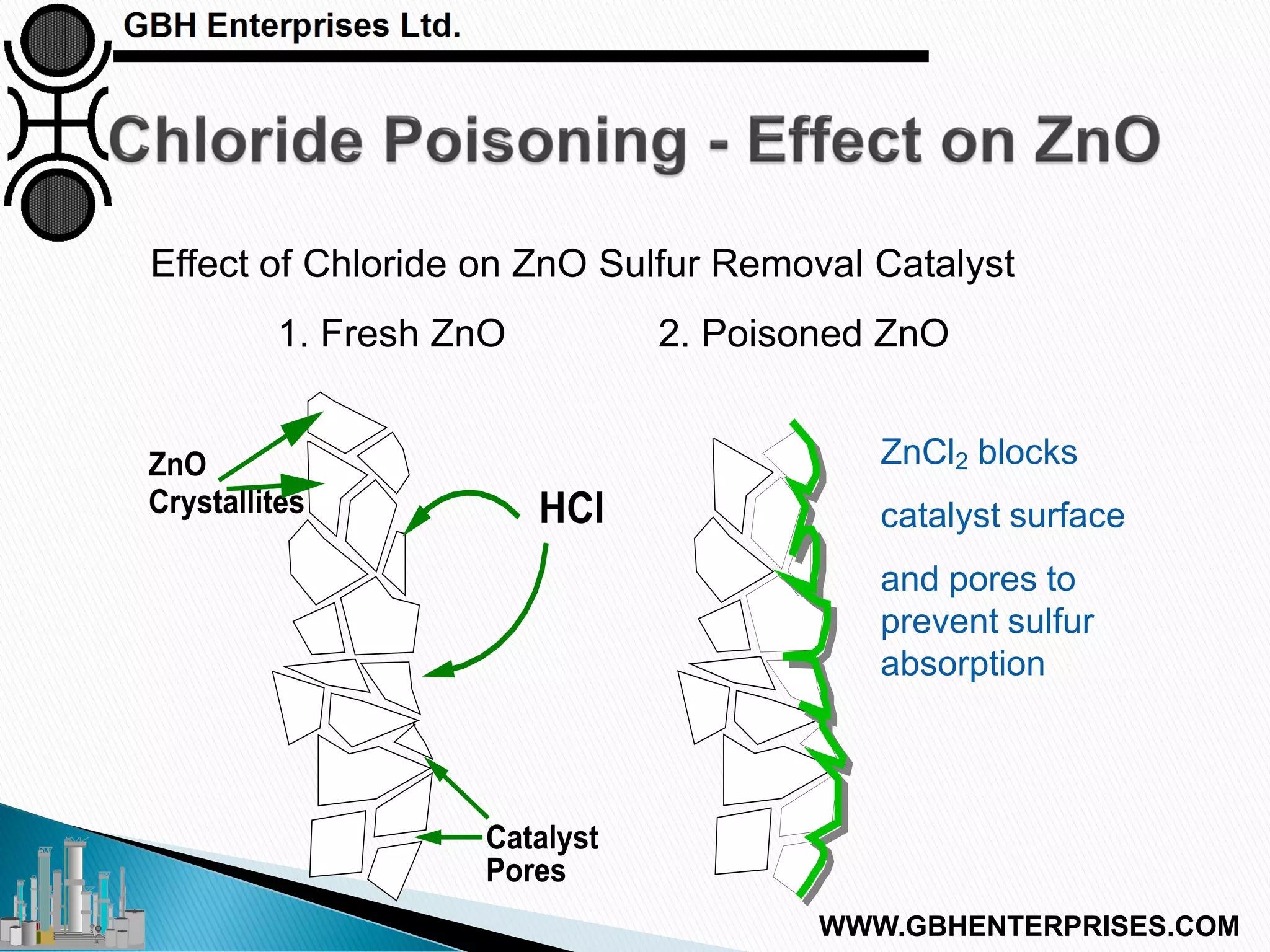
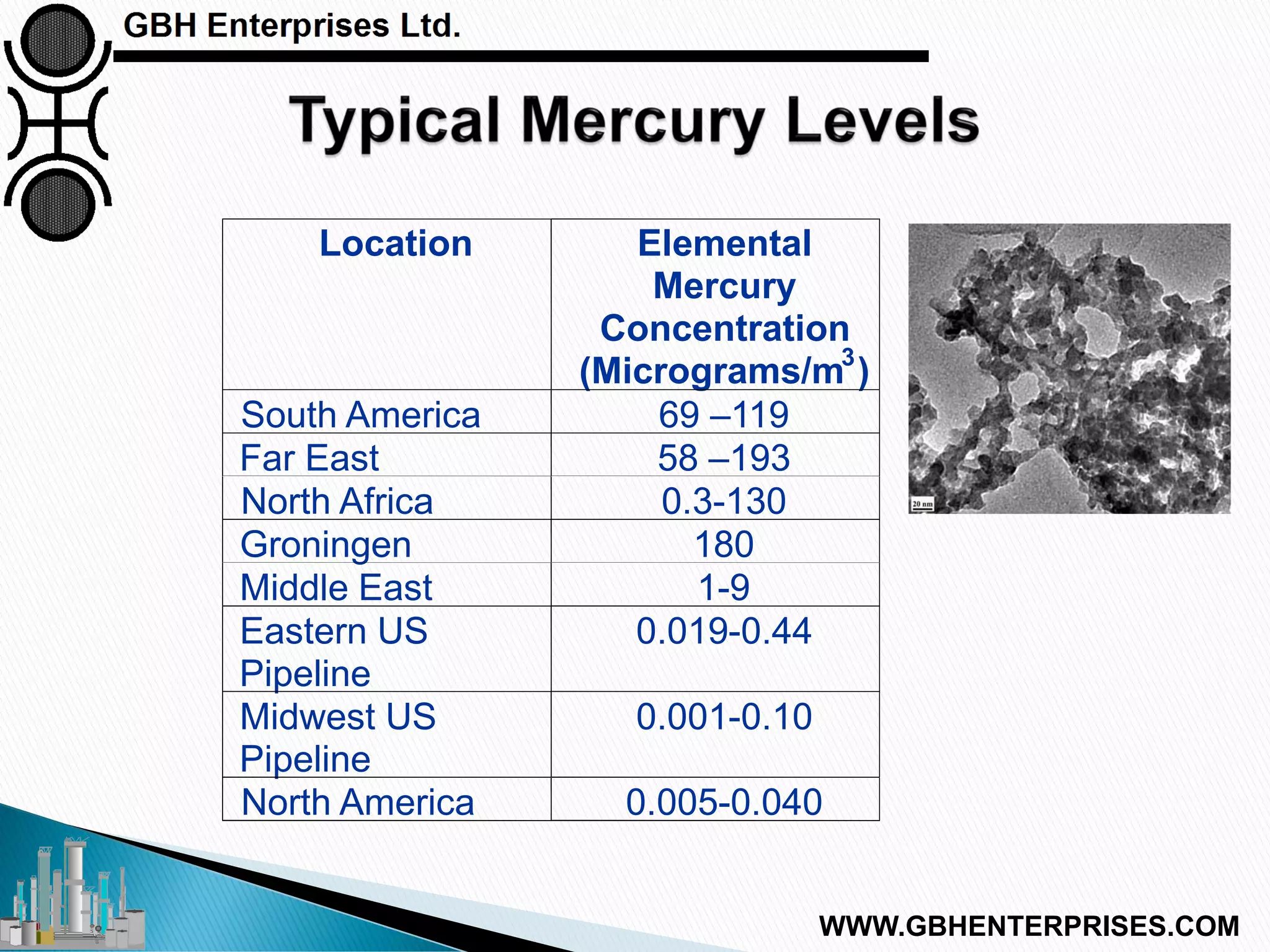
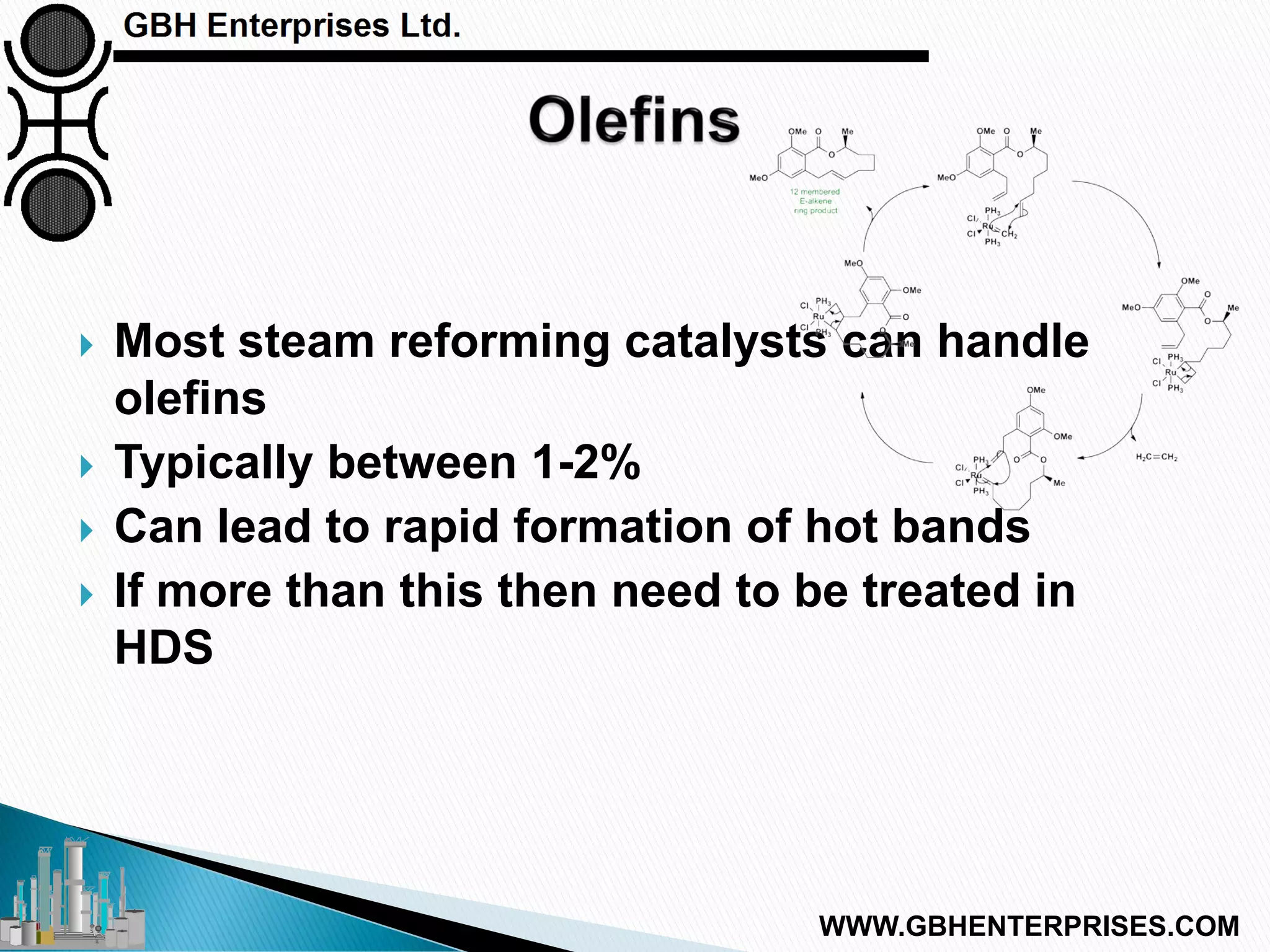
The presentation discusses the various poisons, including sulfur, chlorides, and metals, that can affect steam reforming catalysts, leading to reduced catalyst activity and undesirable reaction temperatures. It outlines specific thresholds for these poisons and the necessary actions to mitigate their impact, such as pre-reforming and using zinc oxide for sulfur removal. Additionally, the document highlights challenges in accurately measuring low levels of these poisons and the effects of different feedstock compositions on catalyst performance.