Rea 1.ppt
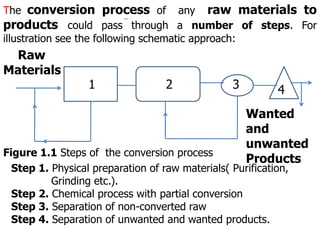
- 1. 1 2 3 4 Raw Materials Wanted and unwanted Products Figure 1.1 Steps of the conversion process The conversion process of any raw materials to products could pass through a number of steps. For illustration see the following schematic approach: Step 1. Physical preparation of raw materials( Purification, Grinding etc.). Step 2. Chemical process with partial conversion Step 3. Separation of non-converted raw Step 4. Separation of unwanted and wanted products.
- 2. In the above processes, the steps 1, 3 and 4 are physical treatments and are mainly carried out in unit operations. Step 2 is the chemical treatment / process / which is carried out in the reactors. The steps of a chemical process can be simple or complex, depending upon the purity of the raw material, the desired quality of the products etc. The main objective of the chemical engineer is to design appropriate reactor and or analysis of performance of chemical reactors to secure quality of the wanted products with a minimum cost of production using the tools of chemical reaction engineering. Chemical reaction engineering (CRE) •is concerned with the rational design and/or analysis of performance of chemical reactors •is a means to determine something about the reactor: size, flow and thermal configuration, product distribution, etc.
- 3. A Chemical reactor is a vessel or a container into which a chemical reaction takes place. The size of such reactor can be a laboratory beaker or an industrial reactor, for example ammonia reactor, which has diameter up to 3 meter and length up to 30 meter. A chemical reactor •is a device in which change in composition of matter occurs by chemical reaction •is a device also involved in energy production, as in engines (internal-combustion, jet, rocket, etc.) and in certain electrochemical cells (lead-acid, fuel), in animate objects (e.g., the human body),etc.. The rational design of this last is rather beyond our capabilities •is usually the “heart” of an overall chemical or biochemical process •is used to carry out the reaction and that is used as a tool for determining something about the reacting system: rate of reaction, and dependence of rate on various factors, such as concentration of species j(cj) and temperature (T)
- 4. •design includes determining the type, size, configuration, cost, and operating conditions of the device The cost of production depends on step 2, i.e., appropriate design of a reactor. To ensure good designing of a reactor in the chemical treatment under step 2, it needs: •Information, knowledge and experience from the different sciences such as fluid mechanics, heat transfer, mass transfer, economics etc. •To answer the following two questions: •What changes can we expect to occur? :-Thermodynamics •How quickly will they take place? :-Kinetics The second question deals with the various rates of processes such as chemical kinetics, heat transfer and mass transfer.
- 5. Thermodynamics Fluid Flow Mathematics Mass transfer Chemical Reactor Heat transfer Materials Economics Kinetics Chemical Process $P
- 6. Generally there are four basic forms of homogeneous chemical reactors which differ from each other in their mixing pattern 1.1. Classification of Reactors Reactors can be classified in a variety of ways: based on the size, method of operation and the phase involved in the process •The batch reactor (BR) •The semi-batch reactor (SBR) •The continuous-stirred tank reactor(CSTR) The plug-flow reactor (PFR Generally the basic reactors are classified
- 7. In the above, all reactors are also named based on the thermal operations such as isothermal, adiabatic and non- isothermal reactors. Most of the industries now a days are working with heterogeneous catalytic reactors, some of them are: •Fixed Bed Gas Reactors •Non-isothermal, Non-adiabatic Fixed Bed (NIHAF) Reactors. •Fixed Bed Gas-Liquid Reactors. oTrickle Bed Reactors oFixed Bed Bubble Reactors(FBBR) •Fixed Bed Reactors •Suspended Bed Reactors. oContinuous Stirred-Tank Reactors(CSTR) oSlurry Reactors • Bulleted Bed Reactors • Three Phase Transport Reactors
- 8. The design procedure for homogeneous and heterogeneous reactors is essentially the same and consists of establishing material balance and energy balance for specific type of reactors involved finding the appropriate rate of equation and solving the required size of the reactor or other parameters 1.2 Design Concept The design of a reactor involves the following concepts: 1.2.1 Steady-State Condition Concept This is a condition, where at the operation the variables at each point within the system do not vary with the time. Therefore, in the reactor the following variables remain unchanged with the time. •The concentration of the reactants and products. •The reaction temperature •The reaction rate
- 9. In steady-state condition, where the above compositions remain unchanged with time. Hence, there is no accumulation. On the other side, this type of operation is simple to model (simple equipment) and accomplish in the case of continuous reactors 1.2.2 Unsteady-State Condition Concept This is opposite to the steady-state condition i.e., the compositions change with time and therefore, accumulations exist. This type of reactor is complicated to model and only used in batch reactor. 1.2.3. Ideal concept in the reactor The contents of the reactor are instantaneously and perfectly mixed i.e., ideally mixed, so that the condition through out the reactor remains the same.
- 10. 1.2.4. Calculation concept Consider the general equation, dD cC bB aA From the equation we select an element to be the basis of calculation, such as A. The base of calculation is most always the limiting reactant. One of the criteria to select the limiting reactant in a single reaction is the cost of the reactant. 1.2.5. Conversion The conversion of species A in a reaction is equal to the number of moles of A reacted per mole of A fed. fed A of moles reacted A of moles A X To understand the concept of conversion, reaction, dD cC bB aA is carried out in a batch reactor under isothermal condition. This is illustrated in Figure 1.1. The conversion obtained through time is calculated in table 1.1.
- 11. nA,0 nA Figure 1.1 Variable in batch reactor t (min) 0 0 10 0.5 20 0.75 30 0.88 40 0.99 0 , A n 2 0 , A n 4 0 , A n 8 0 , A n 16 0 , A n A n A X
- 12. •For Irreversible reactions The maximum value of conversion, XA, is that for complete conversion, i.e., XA = 1.0 •For Reversible reactions The maximum value of conversion, XA, is the equilibrium conversion, i.e., XA = XA,equ. Progress Test 1.1 For the reaction dD cC bB aA Write equilibrium constant in terms of conversion
- 13. 1.2.6 Selectivity Is the formation of the desired product (species j) divided by the formation of all products formed products all of amount formed j product desired of amount j S This is a number that goes from zero to unity as the selectivity improves. We can use the number of moles nj, chosen on some basis for each species such that we divide each nj by its stoichiometric coefficient to normalize them. For a steady- state flow system the molar flow rates Fj are appropriate. If we can identify a reactant A on which to base the selectivity, then we can define the selectivity as F - F F n - n n A A,0 B A A,0 B B S as long as loss of one mole of A can form one mole of B.
- 14. If the system is also at constant density, this can also be written C - C C A A,0 B B S and, for the A B C or A B, A C systems, the selectivity to form B becomes C C C C B B B S 1.2.7Yield in multiple reactions is the amount of the desired product formed divided by the amount of the reactant fed fed reactant formed j product desired j Y For reactant A and product B with one mole of B formed per mole of A reacted, the yield is given by the expression C C F F n n A,0 B A,0 B A,0 B B Y
- 15. Note finally that the yield is always the selectivity times the conversion X S A B B Y Since the conversion is based on a reactant species, the yield is based on a specific product and a specific reactant. For our A B C and A B, A C reaction systems, species A is the reactant, species B is the desired product, and C the undesired product, and there is no change in number of moles ( ) so these expressions become particularly simple, 1 , 2 , 2 , 1 , 1 C B B A A,0 A A,0 A C C - C X C C C C C C A A,0 B C B B B S C C X S A,0 B A B B Y
- 16. Using Differential/ instantaneous/ selectivity, for a single reactant A, the product ratio is, B s A dC B dC C dC B dC B dC Which is the rate of forming B divided by the rate of loss of A or which determines to what extent reactant A being is converted into B at some place or at some moment. This is a useful tool for choosing local reaction conditions in any reactor. Using the above equation, we can formulate: B dC A dC B s And integrating, we obtain 0 , B C A dC A C A C B s
- 17. 1 - C S 0 , 0 , 0 , B B A A C A C B A A A A dC s C C C C For two parallel irreversible reaction with orders α1, α2 becomes k k k r C 0 , 2 2 1 1 1 1 0 , 2 1 1 B A A C A C A A A A A C A C dC C C C dC r r The total selectivity can be found by writing as
- 18. If α1 = α2 , then the integral becomes ) - ( k k k k C 0 , 2 1 1 0 , 2 1 1 B A A A A C A C C C dC k k If α1 = 0 and α2 =1, then this expression becomes A A A C A C A A C k k C k k k k dC C k k k 2 1 0 , 2 1 2 1 0 , 2 1 1 B ln C and the total selectivity is A A A A A A B C k k C k k C C k k C C C 2 1 0 , 2 1 0 , 2 1 0 , B ln ) ( S
- 19. t C m C j m 1.2.8 Throughput (capacity) Feed Rate This is the volumetric or mass flow rate through the reactor system is the mass flow rate of species j Where 1.2.9 Load (Intensity) This is the volumetric or mass flow rate per unit reactor volume or catalyst mass. W t C m V t C m V C I 1 1 1.3 Material Balance see Rxn Eng’g I - Kinetics
- 20. 1.4 Energy Balance A similar word statement as of material balance can be used for energy balance, i.e the application of the principle of conservation of energy leads to an energy balance which is in general states that: Rate of Rate of Rate of Rate of Accumulation = Energy - Energy + Energy of Energy In Out Production In principle, all forms of energy, like heat, kinetic energy, potential energy, electrical and magnetic field must be taken into consideration. In most reactor calculations, the terms with thermal energy and work done on the surroundings are of the main importance. Hence, leaving out the other effect, the energy balance for an open system in which reaction takes place, illustration is given in the Figure 1.2
- 21. HF HE Q dt dH Figure 1.2 Energy balance on an open system: HF – inflow of enthalpy; HE – outflow of enthalpy; Q – rate of heat supply from the surrounding or withdrawn from the system Using equation (1.10) the energy balance for the above open system is dt dH Q E H F H ( 1.10.1) or dt dH T S T KA E H F H ) ( (1.10.2)
- 22. dt RV R H ) ( dT P C T m The enthalpy change should be expressed into two elements: i)The enthalpy change with time due to the change in composition. In another word, the energy change due to the heat of the reaction. ii) The enthalpy change due to the change of temperature If we now substitute the enthalpy change to equation (1.10.2), we obtain dt dT P C T m dt rV R H T S T KA E H F H ) ( ) ( or dT P C T m dt rV R H dt T S T KA dt E H F H ) ( ) ( ) ( Rearranging the equation, we can now write the general energy balance for the system ) ( ) ( ) ( T S T KA E H F H rV R H dt dT P C T m
- 23. where K-Overall heat transfer coefficient TS-Surrounding (cooling, heating) temperature A- Effective area for heat transfer T- The temperature of the reaction mixture ∆HR - Heat of reaction To put this general energy balance equation in a more applicable form, there are two conditions for modification: In a batch process where there is no in- and out-flow, then the energy balance equation yields to ) ( ) ( T S T KA rV R H dt dT P C T m To put this general energy balance equation in a more applicable form, there are two conditions for modification: In a continuous process where accumulation is not existing, the energy balance equation then becomes ) ( T S T KA dH E H F H
- 24. ) (rV R H dT P C R F dH ) ( ) ( T S T KA rV R H dT P C R F where Then, the enthalpy balance for continuous process is (1.12) (1.13) 1.5 Designing of a Reactor In a reactor design, the main parameters are to choose the type of reactor, to select the method of operation and to find the size of the reactor. In this selection, common types of reactors and operation methods are known. Therefore, the choice of reactor would be made on the basis of profit, safety and environmental factors which will influence the reactor performance. Sizing of a reactor means to determine the reactor volume necessary to achieve a specified conversion. The condition in the reactor varies with position as well as with the time and therefore, it is necessary to find the rate equation.
- 25. Normally the rate of reaction is expressed in terms of the concentration, but not in terms of conversion. However, we need to express the concentration of the reacting species in terms of conversion, for which we use stoichiometry relationship for batch and flow processes. 1.5.1 Batch Process - see Rxn Eng’g I- Kinetics 1.5.2 Flow Process - see Rxn Eng’g I- Kinetics 1.6 Conversion in multiple reactions - see Rxn Eng’g I- Kinetics