Embed presentation
Downloaded 18 times



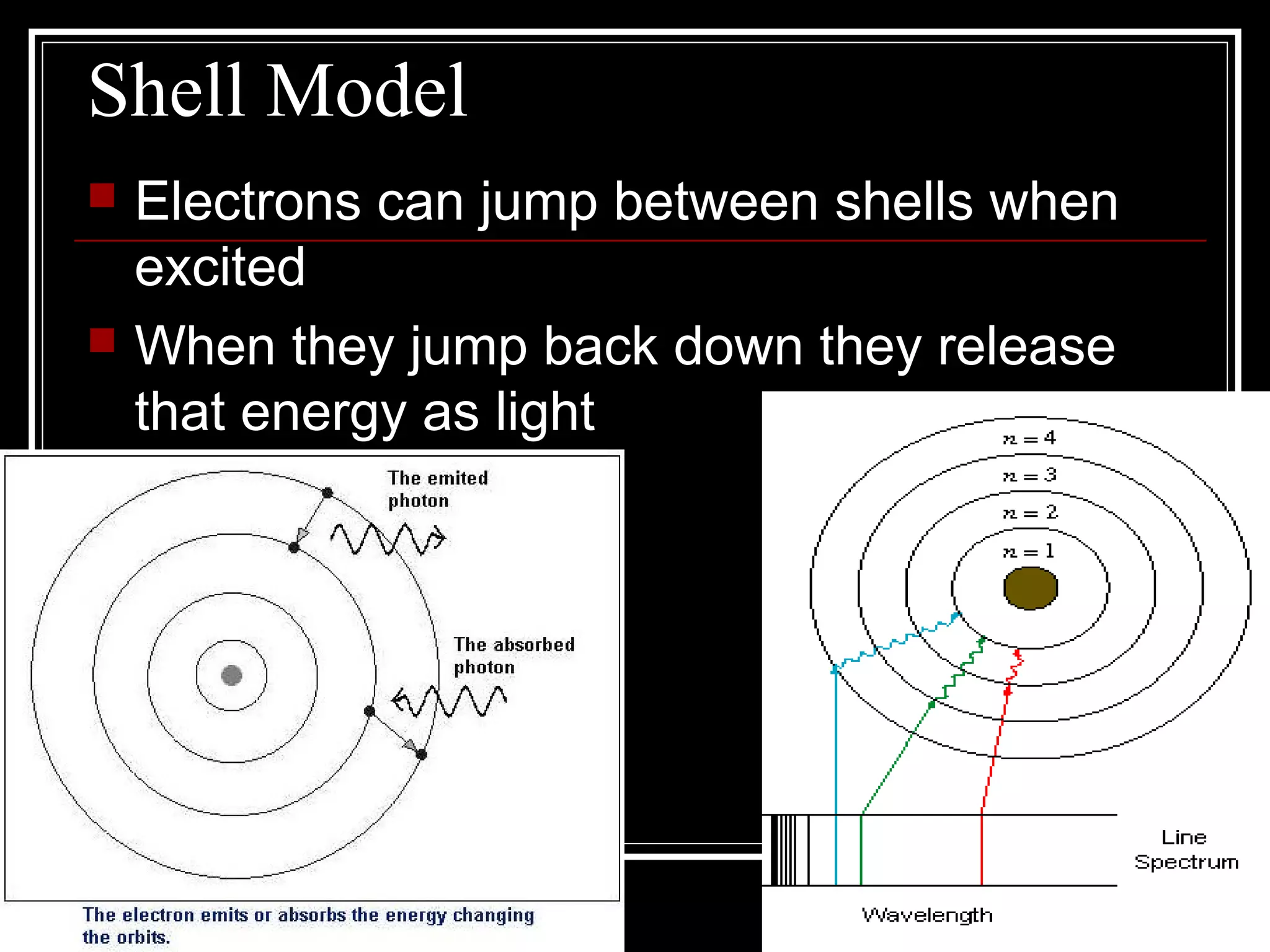


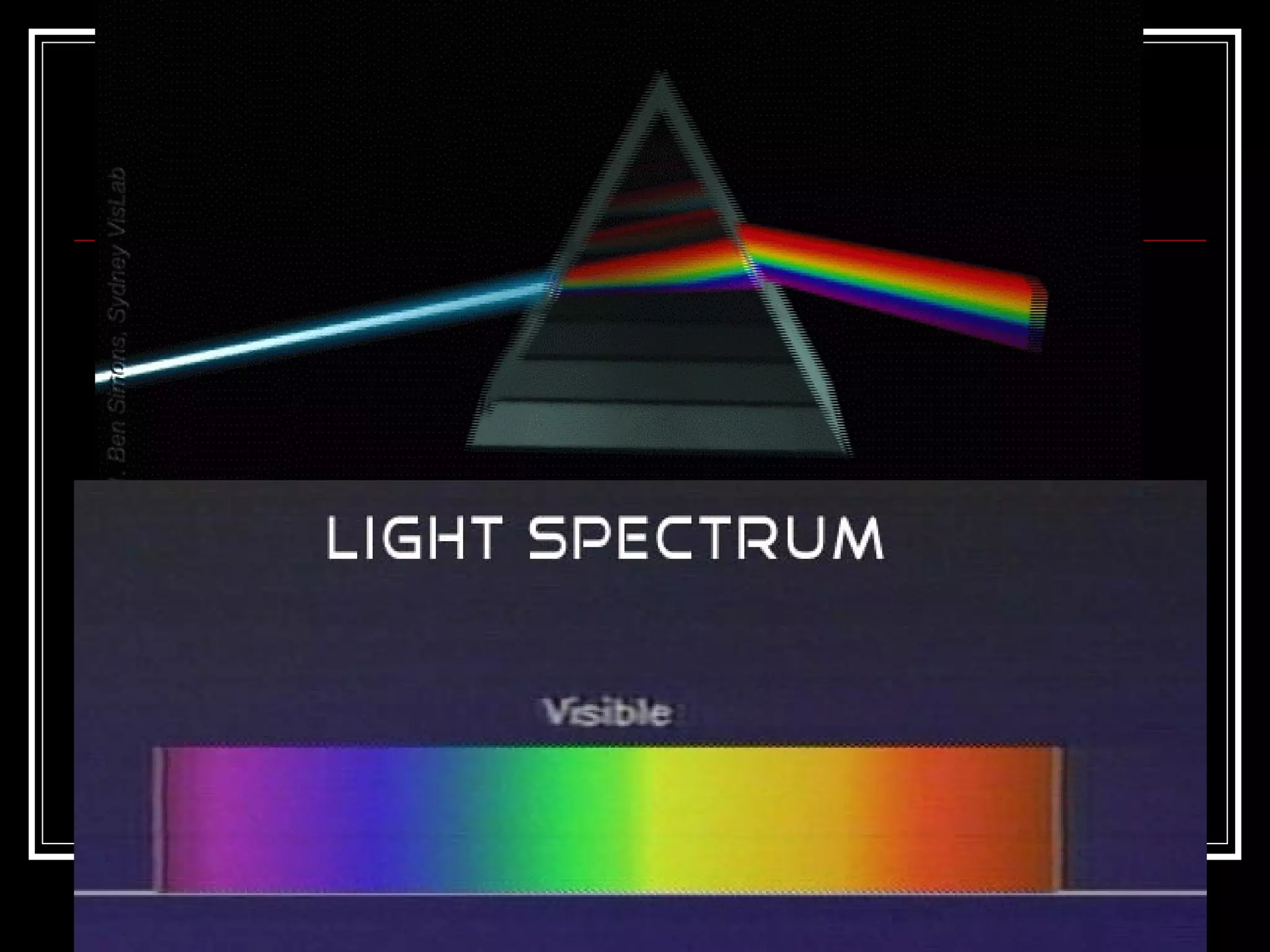


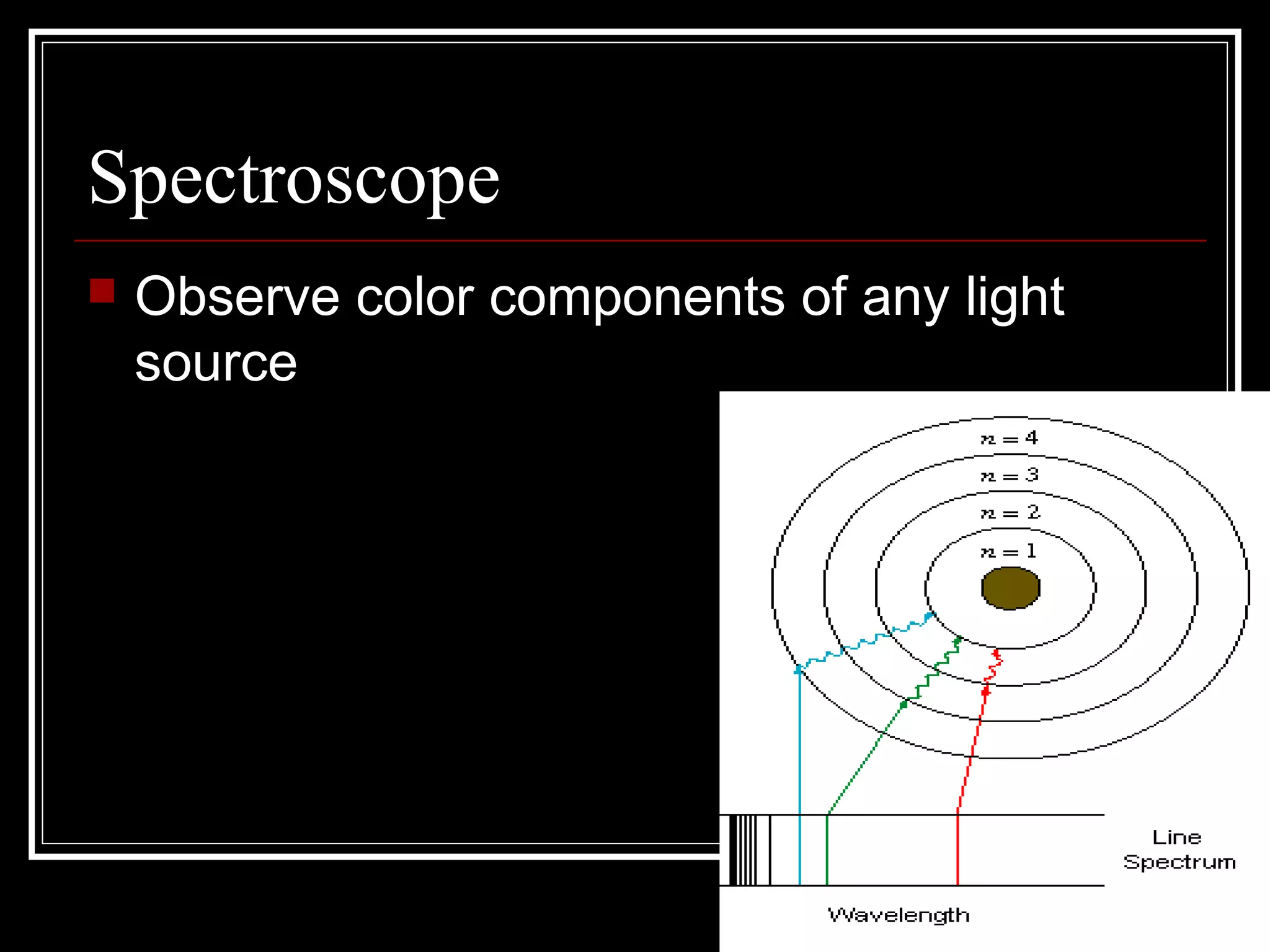
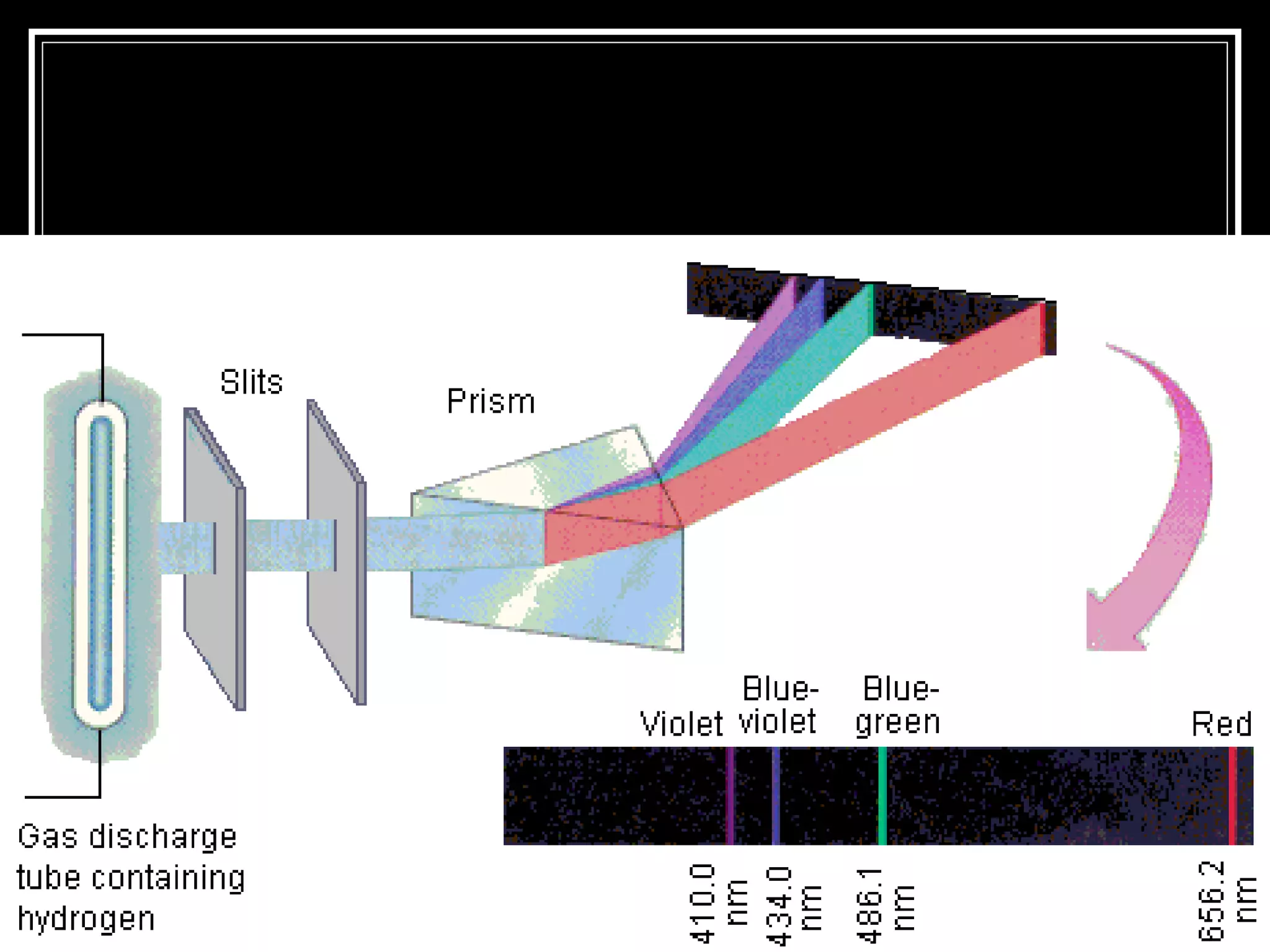

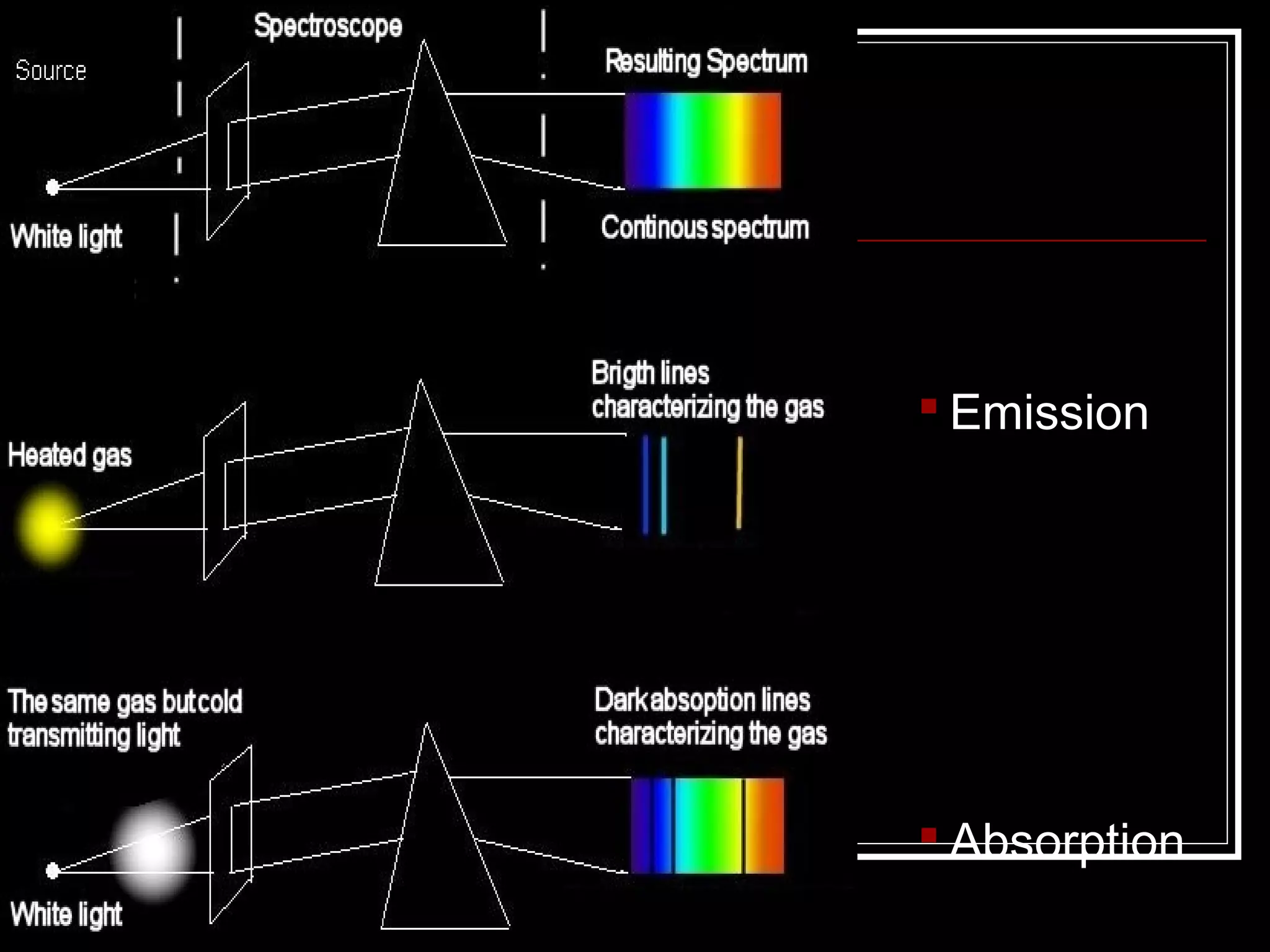


This document discusses atomic spectra and how they can be used to identify elements. It explains that atoms emit and absorb light at specific frequencies when electrons jump between energy levels, and that each element has a unique atomic spectrum. A spectroscope can be used to separate light into its component frequencies, allowing scientists to determine the chemical composition of stars and perform flame tests by observing the atomic spectra emitted or absorbed.















