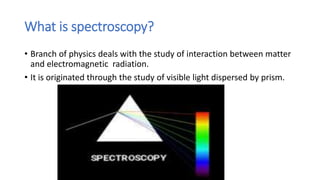
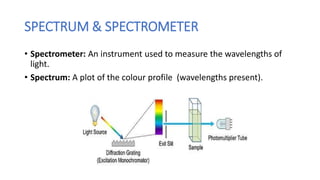
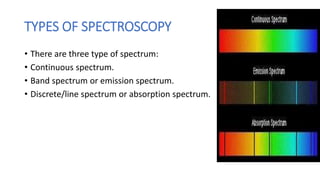
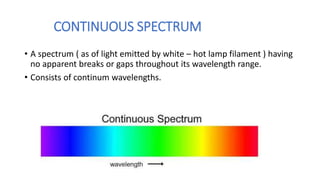
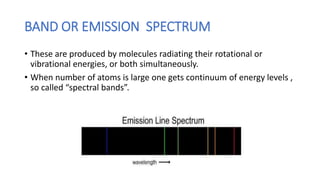
This document discusses spectroscopy and atomic spectra. Spectroscopy is the study of interaction between matter and electromagnetic radiation. There are three main types of atomic spectra: continuous spectra which have no gaps in wavelengths, band or emission spectra produced by molecules radiating energy in rotational or vibrational transitions, and discrete line spectra seen in isolated atom absorption and emission at particular wavelengths. Atomic spectra result from electrons transitioning between quantized energy levels of an atom when excited, and hydrogen emits characteristic wavelengths that form different spectral series depending on the electron transition.