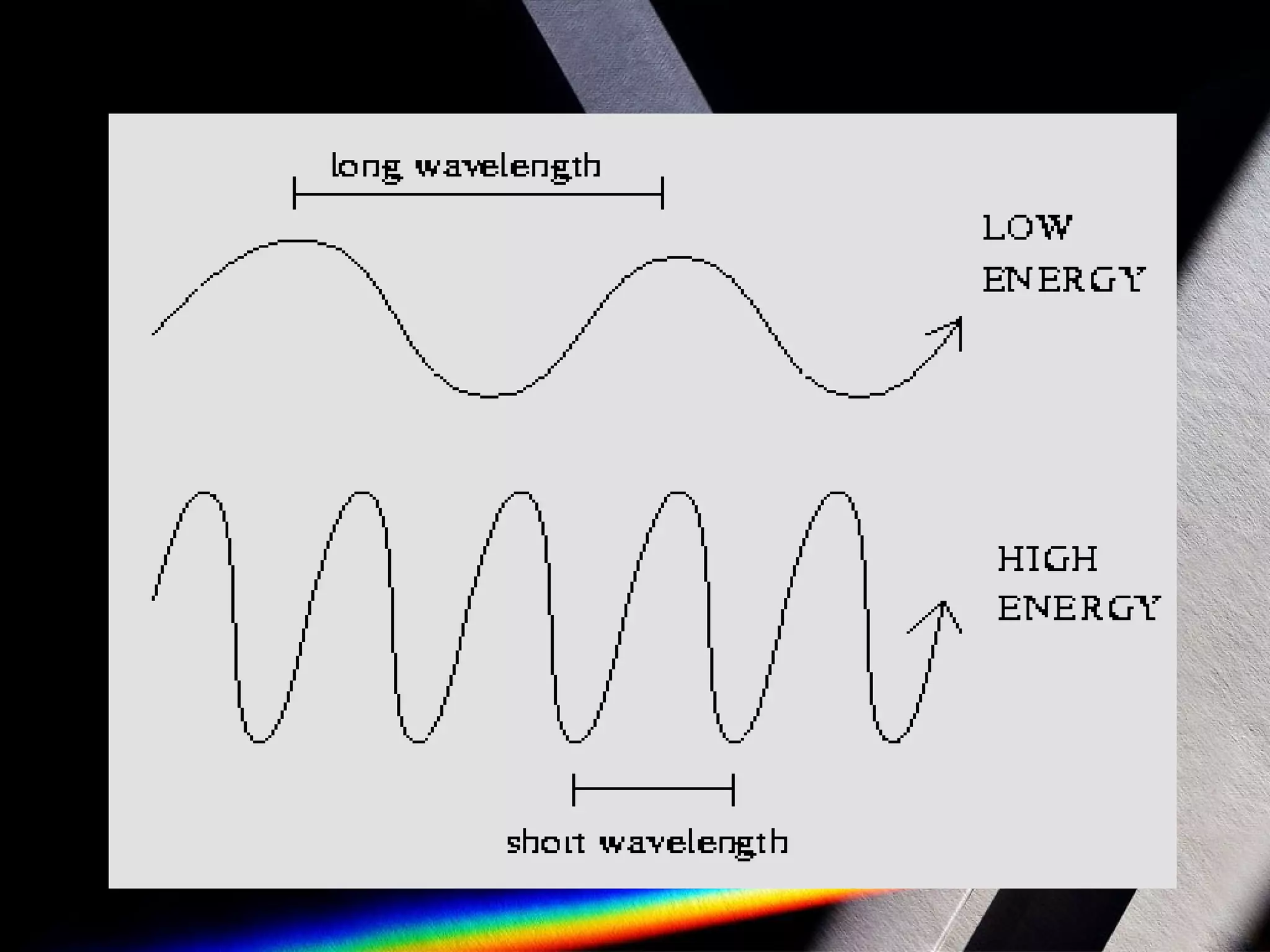
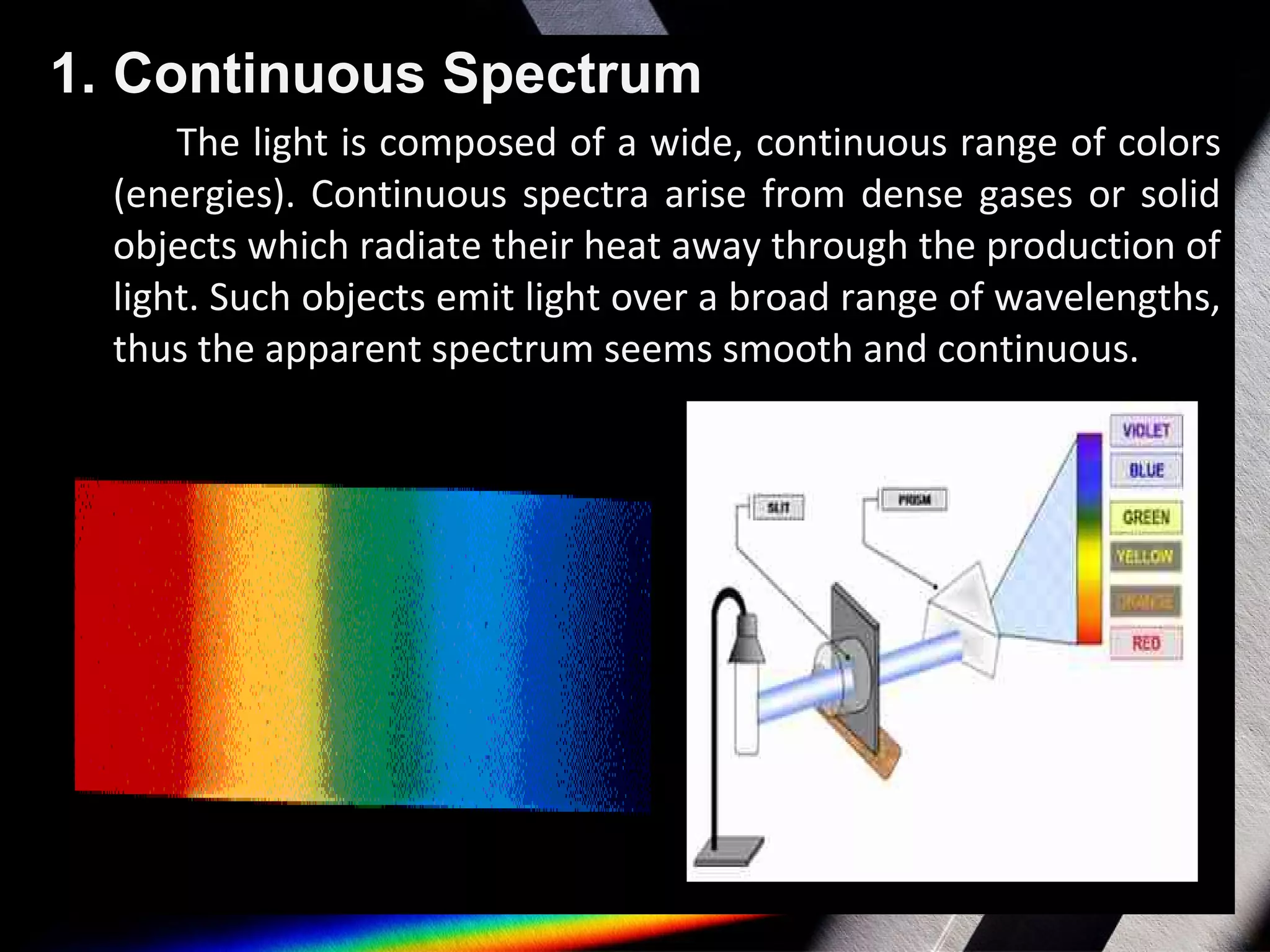
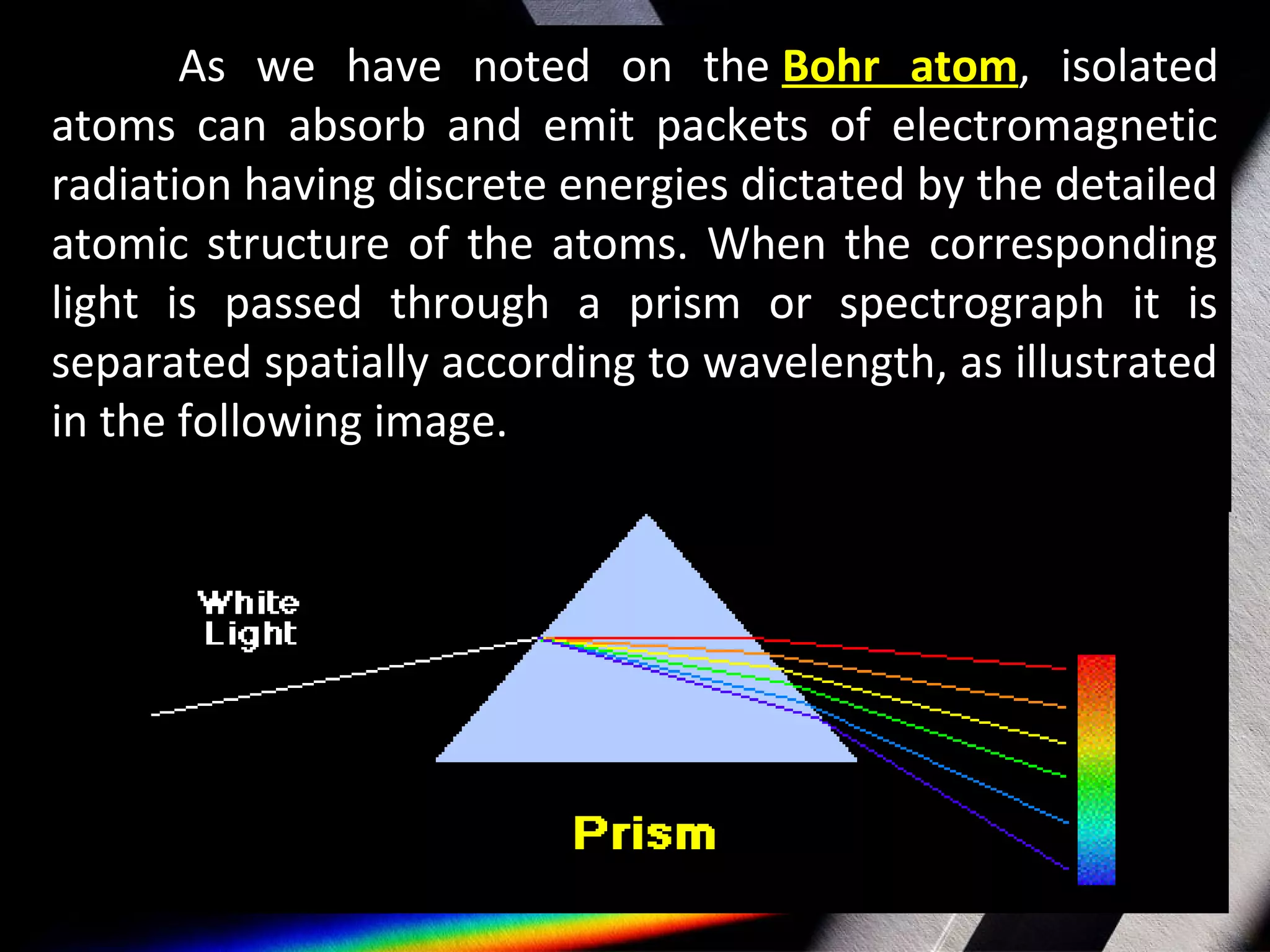
Spectroscopy is the study of light in different wavelengths. It involves dispersing an object's light into its component colors to infer physical properties like temperature, mass, and composition. There are three main types of spectra: continuous spectra from hot gases/solids with a wide range of wavelengths, absorption line spectra which appear as dark lines on a continuous spectrum where light passes through cool gas, and emission line spectra which are bright lines corresponding to photons emitted when excited atoms in a gas relax to lower energy states. Atomic spectroscopy has applications like identifying elements based on their unique emission spectra and discovering new elements by analyzing absorption spectra from celestial objects.