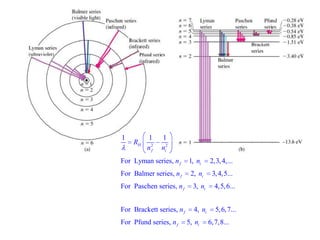
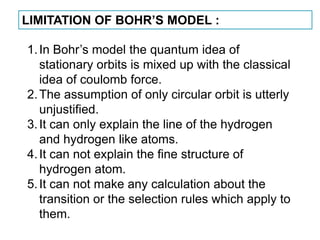
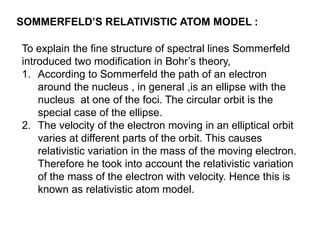
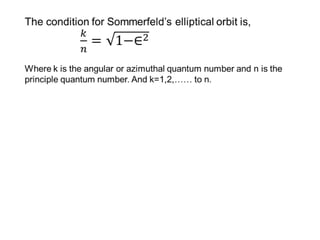
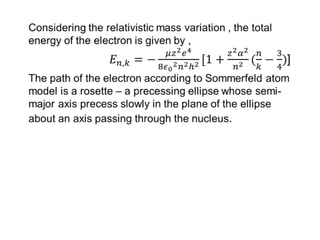
This document provides a history of atomic structure models from ancient Greek philosophers to modern physics. It summarizes Democritus' idea of indivisible atoms, Dalton's billiard ball model of atoms, Thomson's plum pudding model, and Rutherford's nuclear model based on his gold foil experiment. It then describes Bohr's planetary model with quantized electron orbits and energy levels. Later models like Sommerfeld's addressed limitations like explaining hydrogen's fine structure. The document traces how atomic structure models evolved as new experimental evidence emerged.








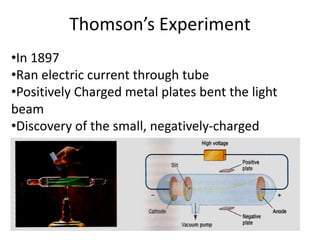
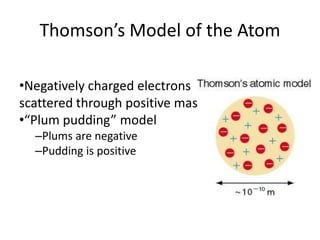


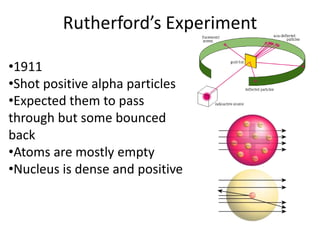
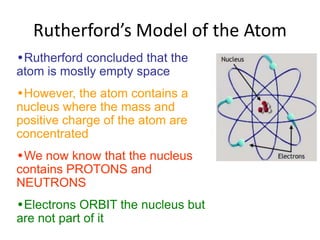
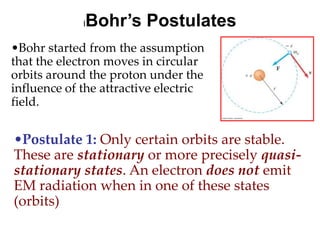
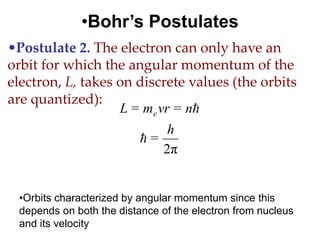
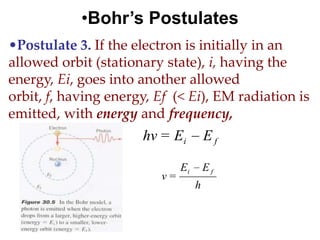
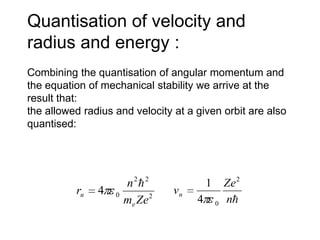
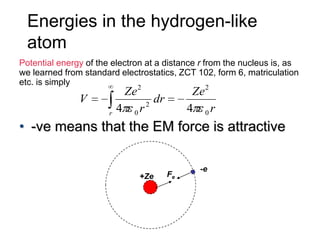
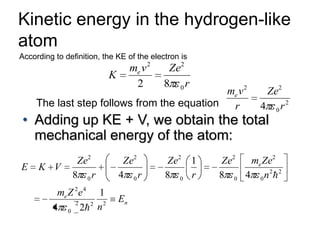

![01.aditya atomic models [repaired]](https://image.slidesharecdn.com/01-aditya-atomicmodelsrepaired-120417105421-phpapp01/85/01-aditya-atomic-models-repaired-29-320.jpg)