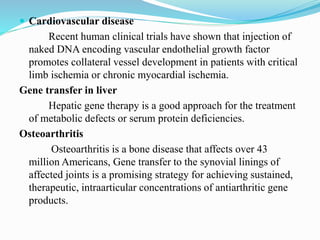
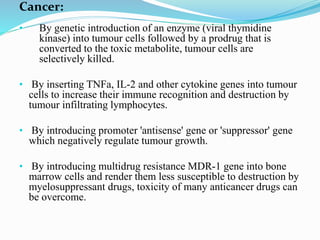
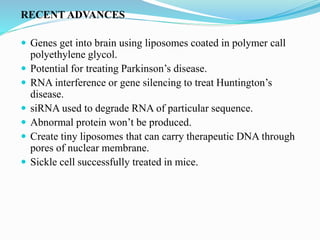
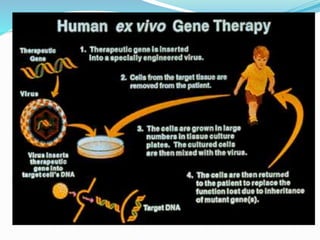
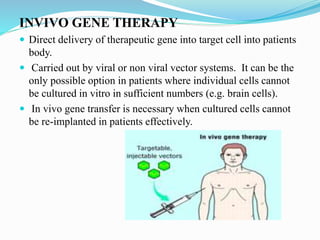
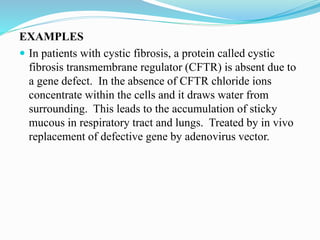
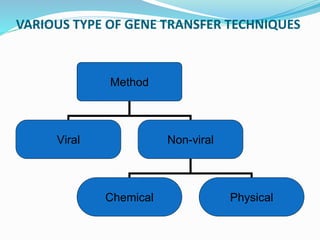
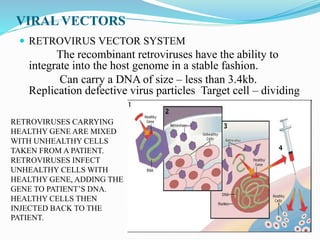
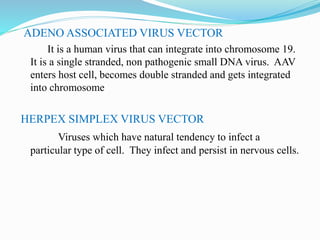
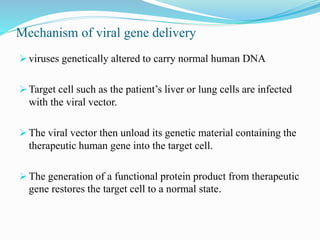
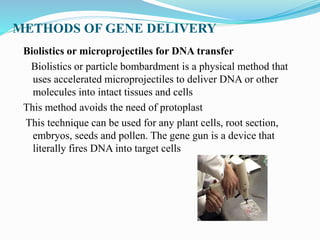
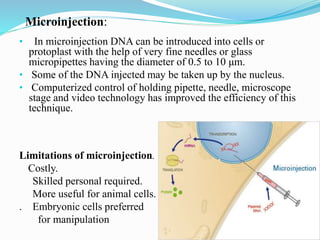
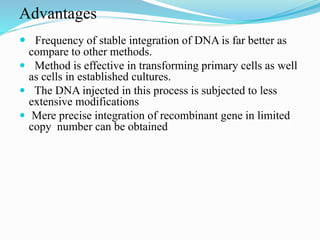
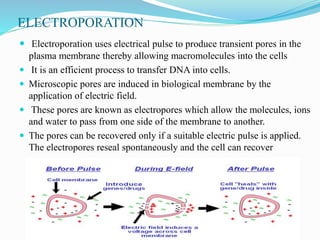
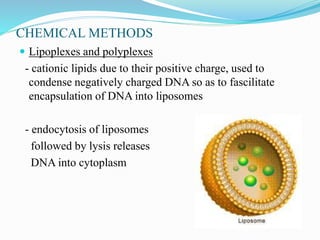
Gene therapy involves introducing genes into cells to treat or prevent disease. It works by correcting defective genes that cause disease or by making cells produce products to treat the disease. The first approved gene therapy treated a girl for ADA-SCID. There are two main approaches - in vivo therapy directly delivers genes into body cells, while ex vivo therapy transfers genes to cultured cells before reinsertion. Viral vectors like retroviruses and adenoviruses are often used due to their ability to deliver genes, but come with risks like insertional mutagenesis. Non-viral methods include physical methods like microinjection and chemical methods using liposomes. Gene therapy shows promise for diseases like cancer, cardiovascular disease, and neurological disorders.


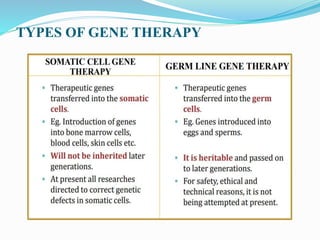




![Applications of Gene Therapy
Severe combined immunodeficiency disease (SCID)
Growth hormone deficiency: by implanting cultured myoblasts
transfected with GH gene.
Familial hypercholesterolemia: by introducing LDL receptor
gene into hepatocytes.
Lesch-Nyhan syndrome: by introducing HPRT gene.
Parkinsonism
It has significantly improved the weakness of the symptoms such
as tremors, motor skill problems, and rigidity
Done with local anesthesia, used a harmless, inactive virus [AAV-
2 ]](https://image.slidesharecdn.com/genetherapy-200428174558/85/Gene-therapy-26-320.jpg)