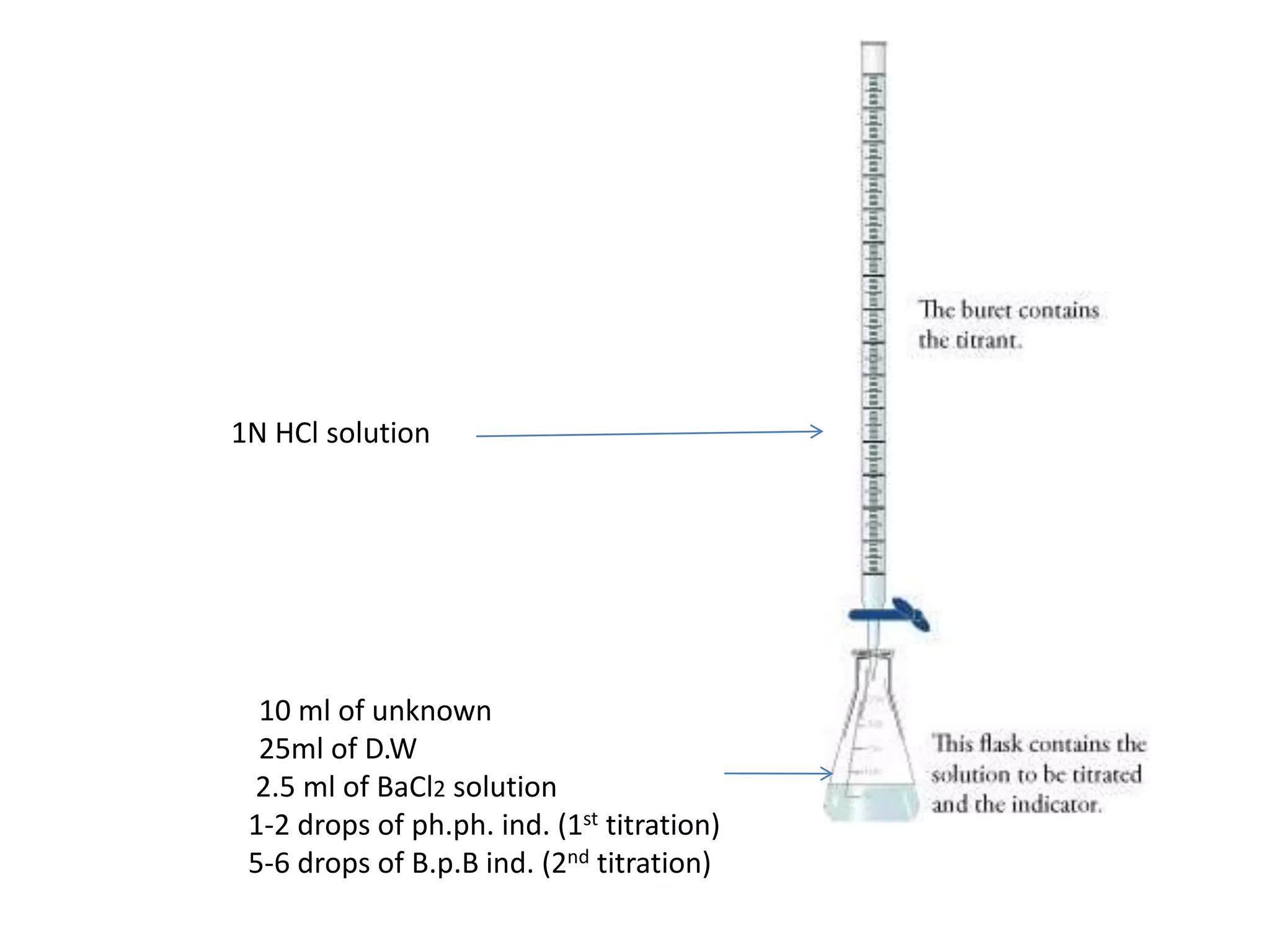
The document details the assay procedure for sodium hydroxide (NaOH) solution, which must contain at least 97.5% w/w total alkali as NaOH and no more than 2.5% w/w Na2CO3. It describes a two-step titration process using hydrochloric acid (HCl) to assess total alkalinity and the chemical reactions involved. Additionally, it outlines the calculations for determining the chemical factors for NaOH and Na2CO3 related to their equivalents with HCl.