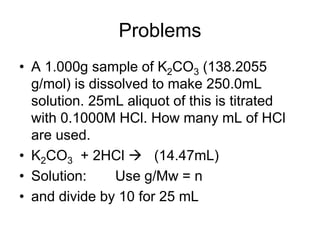
This document provides an introduction and overview of the aims and outcomes of a laboratory skills course, including developing lab skills, independent work, good lab habits, honesty, organization, and problem solving. It discusses specific skills like noticing errors, using units, understanding lab management systems, and health and safety. It then focuses on titration techniques, including the stoichiometry, equipment, and theory involved in volumetric analysis and using indicators to determine the equivalence point of a titration reaction. Examples of titration calculations are provided to determine unknown concentrations. The document emphasizes the importance of units and stoichiometry in analytical measurements and calculations.