The document describes a procedure to determine the alkalinity of a water sample. Alkalinity is measured by titrating the sample with a strong acid like HCl or H2SO4 until the pH reaches certain values. The titration is done in two stages - phenolphthalein alkalinity until pH reaches 8.3, and methyl orange alkalinity until pH reaches 4.5. The total acid volume used is used to calculate the alkalinity in terms of mg/L CaCO3. The procedure involves preparing reagents, standardizing the acid, titrating sample water and making calculations based on the reactions of carbonate and bicarbonate with acid.


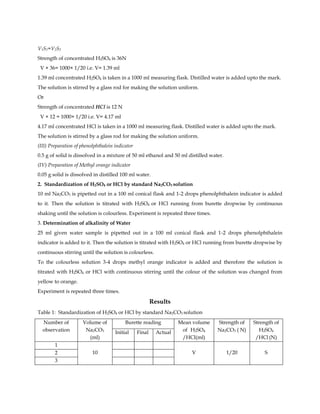

![For using HCl
CaCO3+ 2HCl= CaCl2 + H2CO3
Here, 2 equivalents CaCO3 reacts with 2 equivalents HCl i.e. 1 equivalent CaCO3 reacts with 1 equivalent
HCl.
Hence, 1000 ml 1(N) HCl= 1000 ml 1 (N) CaCO3
1000 ml 1(N) HCl = 50 g CaCO3
Z ml S (N) HCl = (50×Z×S)/1000 g
25 ml water contains (50×Z×S)/1000 gm CaCO3
Therefore, 1000 ml water contains= (50×Z×S)/25 g CaCO3
Conclusion
The alkalinity of water is…………………….. g/L of CaCO3
Viva Voce
1. What is alkalinity? How is it expressed?
2. What is the cause of alkalinity in water?
3. How can you determine the alkalinity of water? Explain with reactions.
4. Concentrated HCl and concentrated H2SO4 are secondary standard. Explain.
Answer. HCl and H2SO4 are both commercially available as concentrated solutions which are easily
diluted and hence concentration of concentrated solution is not accurately known. Moreover, HCl is a
gas and concentrated HCl is prepared by dissolving HCl gas in water. Hence HCl is volatile and
strength of HCl changes with time.
5. Presence of both OH- and HCO3
- is not possible in water. Why?
Answer. Presence of both OH-, HCO3
- is not possible in sample water, since they combine
together to form CO3
2- ions.
OH-+ HCO3
- CO3
2-+ H2O
Alkalinity of sample water
= [(50× Total volume of acid required for titration of sample water× strength of acid)/ volume of sample water taken] gm
CaCO3/L](https://image.slidesharecdn.com/alkalinityofgivenwatersample-230329075444-0539e58d/85/Alkalinity-of-given-water-sample-pdf-5-320.jpg)