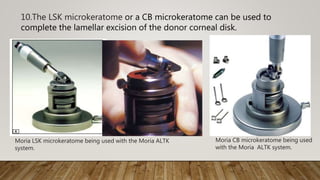
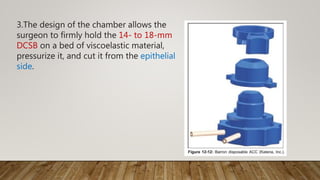
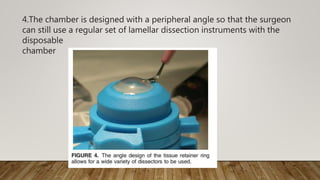
This document describes different types of artificial anterior chambers used in corneal transplantation surgeries. It discusses reusable chambers like the Moria AAC and disposable chambers like the Barron Disposable AAC. The Moria ALTK system allows adjustment of the diameter of the donor corneal resection, while the Barron AAC maintains pressure on the donor cornea during lamellar dissection or trephination. Both systems involve placing the donor cornea on the chamber, adjusting intrachamber pressure, and then performing the corneal resection.