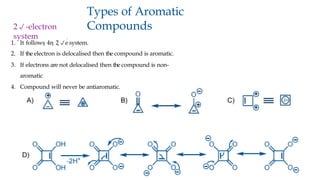
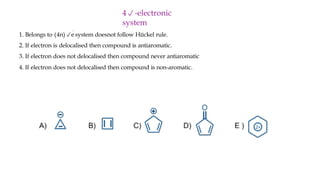
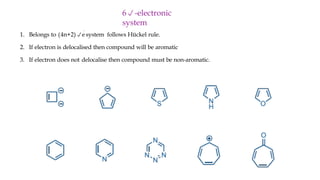
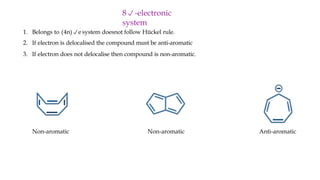
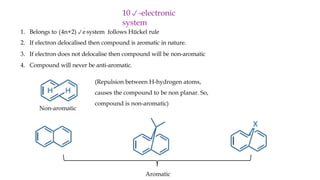
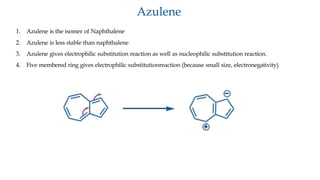
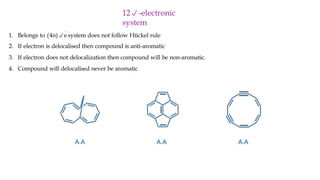
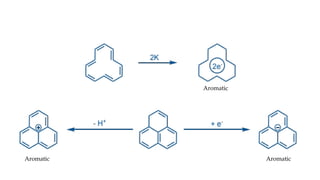
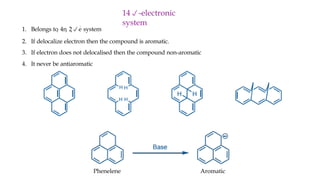
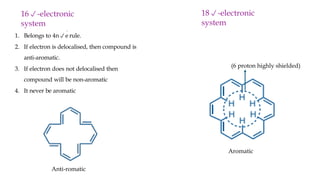
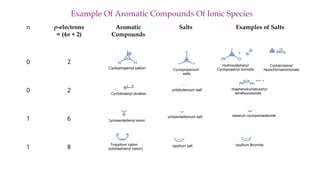
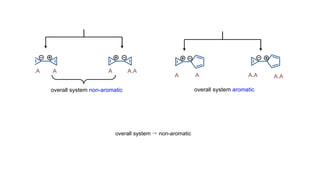
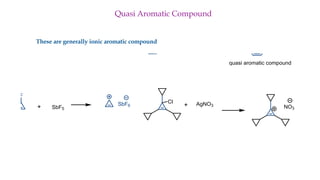
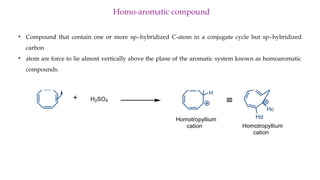
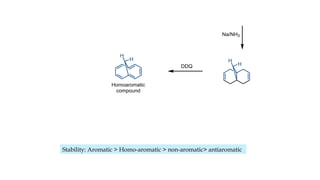
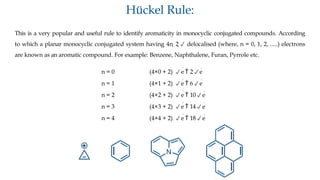
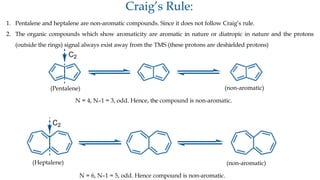
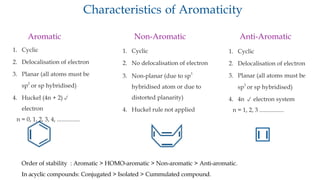
The document outlines the concept of aromaticity in organic compounds, highlighting the criteria for aromatic compounds including stability, resonance energy, and compliance with Huckel's rule (4n + 2 π electrons). It distinguishes between aromatic, anti-aromatic, and non-aromatic compounds, detailing their electronic configurations and magnetic properties. Additionally, it describes various types of aromatic systems, examples of such compounds, and concepts like homo-aromatic and meso-ionic compounds.

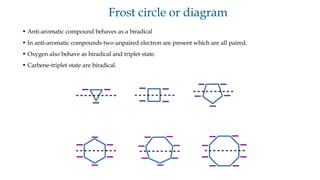
![Frost circle or diagram
1. Energy level of aromatic and anti-aromatic compounds can be shown by usingfrost circleor diagram.
2. Energy levels of regular planar polygonic molecules with an even number of atoms form a
symmetricalpattern
as shown below.
If all bonding energy levels are filled then it is aromatic [Hückel criterion also required].
Antibonding energy level
Bonding energy level
E Non-bonding energy level](https://image.slidesharecdn.com/aromaticity-241107123604-22fff003/85/aromaticity-basics-of-aromatic-compounds-8-320.jpg)