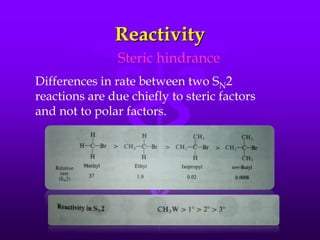
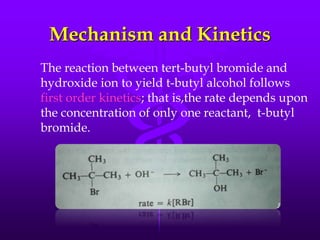
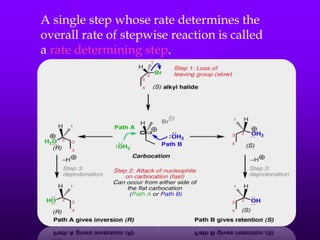
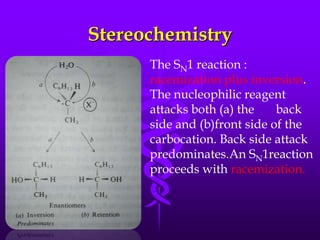
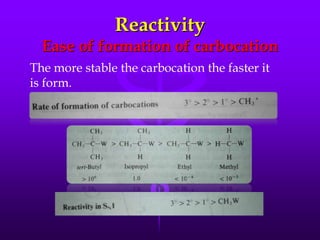
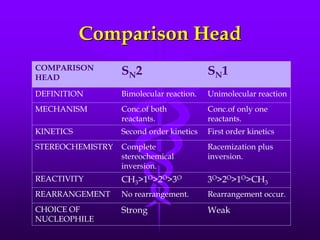
This document provides information on SN1 and SN2 reactions, including definitions, mechanisms, kinetics, stereochemistry, reactivity, and comparisons. SN2 reactions are bimolecular, follow second-order kinetics, and result in complete stereochemical inversion. SN1 reactions are unimolecular, follow first-order kinetics, and result in racemization plus inversion through a carbocation intermediate. Key differences between the two include their order of kinetics, effects on stereochemistry, and choice of nucleophile.