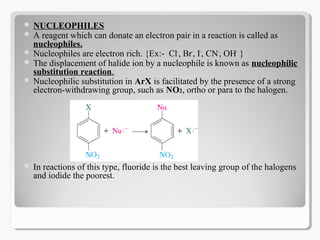
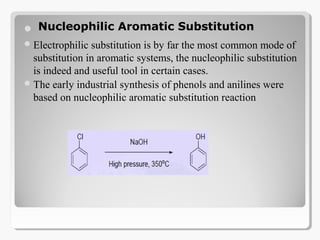
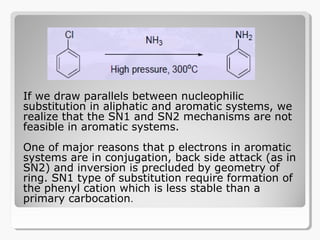
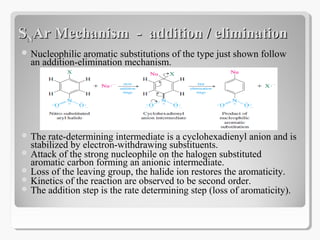
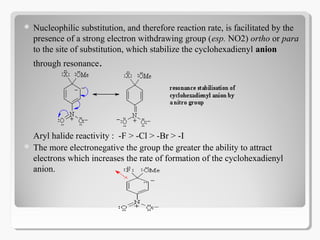
Nucleophilic aromatic substitution reactions follow an addition-elimination mechanism known as SNAr. The rate-determining step is the formation of a cyclohexadienyl anion intermediate through nucleophilic attack. Electron-withdrawing groups stabilize this intermediate through resonance, making the reaction faster. Reactivity decreases in the order of aryl fluorides, chlorides, bromides, and iodides as the leaving group ability decreases in that order. Common nucleophiles like hydroxide, cyanide, and halides attack the aromatic ring.