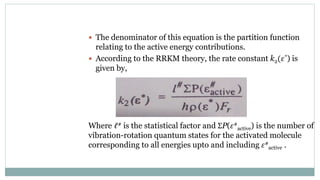
This document summarizes Rice-Ramsperger-Kassel-Marcus (RRKM) theory, which describes the rates of unimolecular reactions. RRKM theory originated from Lindemann-Christiansen theory and considers how energy is distributed among vibrational modes. During the 1950s-1952, R.A. Marcus merged transition state theory with RRK theory. RRKM theory accounts for individual vibrational frequencies and classifies energy as either active or inactive. It can explain high pre-exponential factors and has been used to study reactions like isomerization, though limitations include uncertainty in activated complex vibrational frequencies.