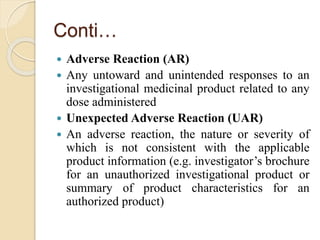
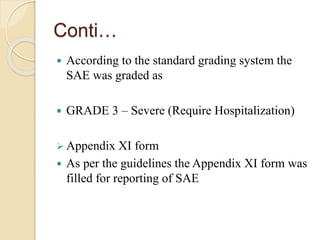
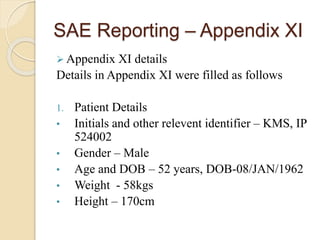
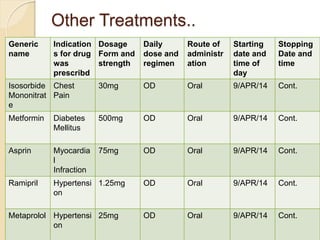
The document discusses adverse event and serious adverse event reporting in clinical trials. It provides definitions for key terms like adverse event, serious adverse event, and unexpected adverse reaction. It outlines ICH-GCP guidelines for safety reporting, including immediate reporting of SAEs to sponsors. Finally, it presents a case study describing a subject's experience of accelerated hypertension as an SAE during a clinical trial and the appropriate reporting steps taken.