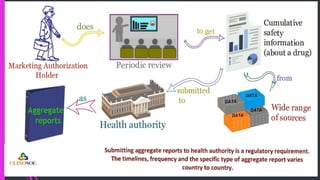
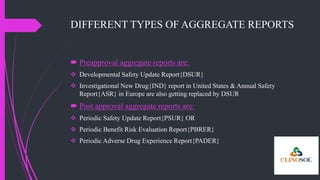
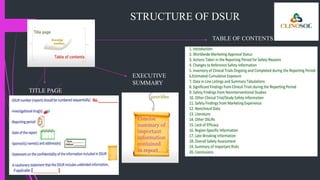
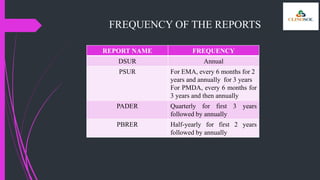
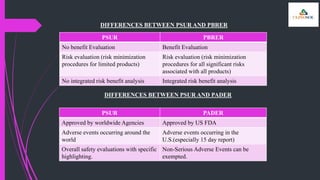
The document outlines various types of aggregate safety reports, including preapproval reports like developmental safety update reports (DSUR) and post-approval reports such as periodic safety update reports (PSUR) and periodic benefit-risk evaluation reports (PBRER). It details the objectives, structures, and reporting timelines for these documents, which aim to evaluate the safety and risk-benefit balance of medicinal products throughout their lifecycle. Additionally, it highlights the frequency of submissions required for each report type and provides comparative insights between them.