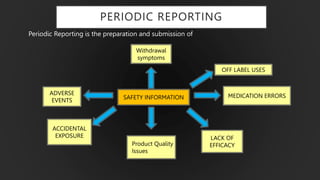
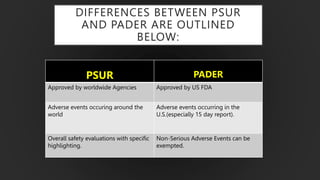
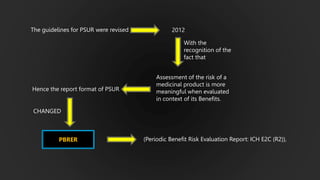
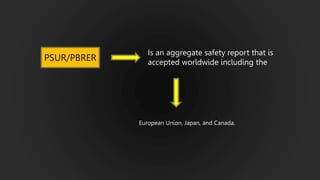
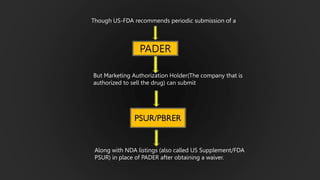
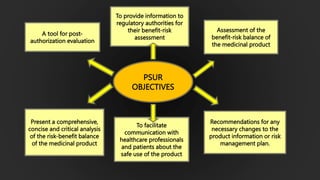
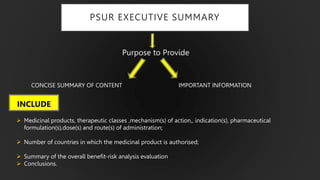
The document details the Periodic Safety Update Report (PSUR), focusing on its objectives, principles, and differences from other periodic reports like PADER and PBRER. It outlines the structure and requirements for PSUR submissions, emphasizing the need for regular updates based on the latest safety data. Additionally, it discusses the importance of PSUR in pharmacovigilance for maintaining drug safety and regulatory compliance.