Embed presentation
Downloaded 26 times
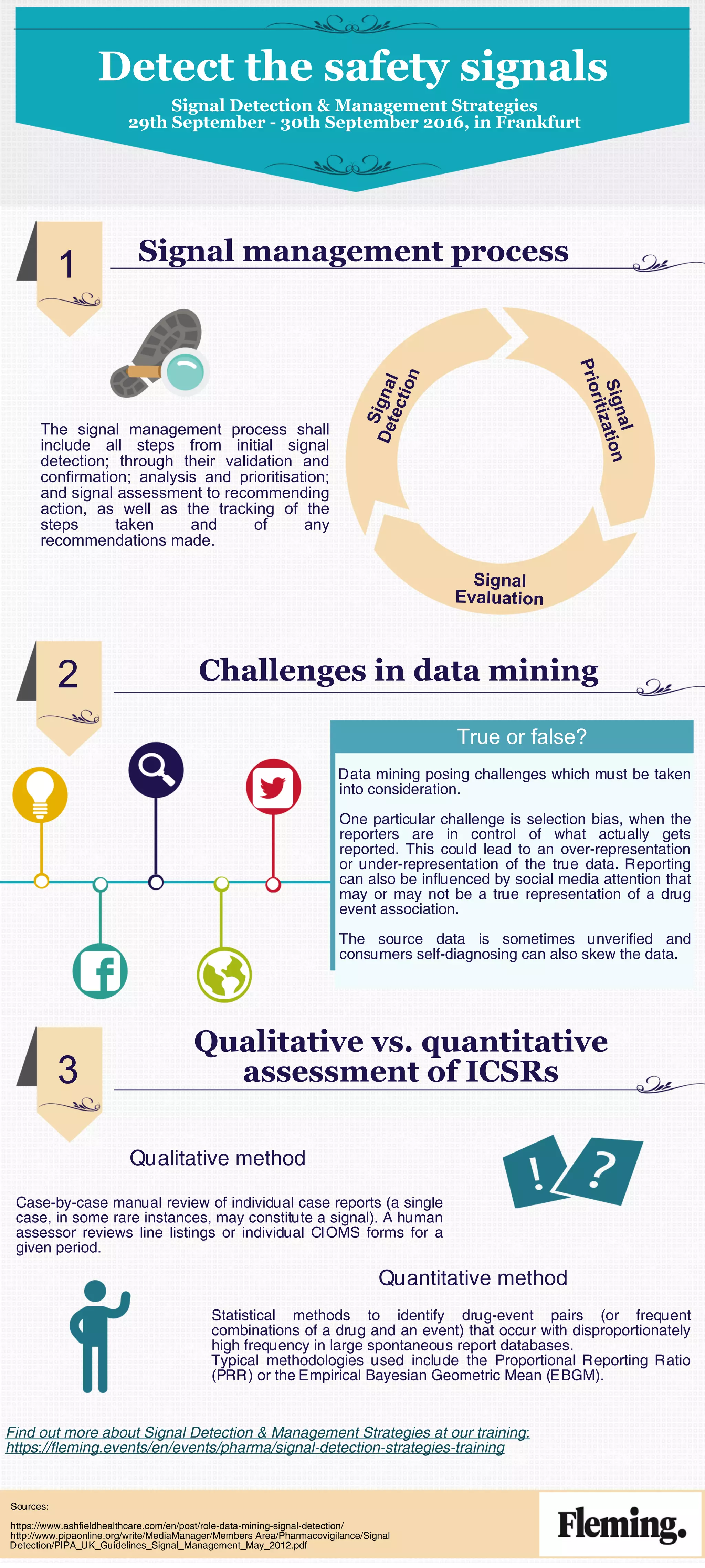

The document outlines the signal management process including detection, validation, analysis, and tracking of safety signals in pharmacovigilance from September 29 to 30, 2016, in Frankfurt. It highlights challenges in data mining, such as selection bias and the influence of unverified data sources, and compares qualitative and quantitative assessment methods for Individual Case Safety Reports (ICSRs). Additionally, it references statistical methodologies like the proportional reporting ratio and the empirical Bayesian geometric mean for identifying drug-event associations.
