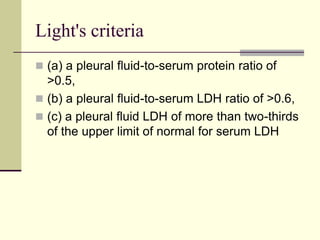
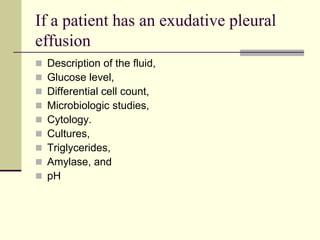
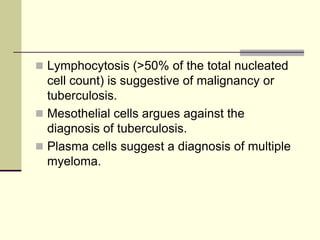
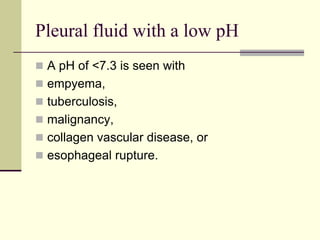
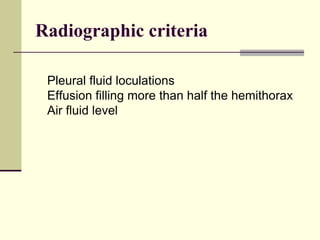
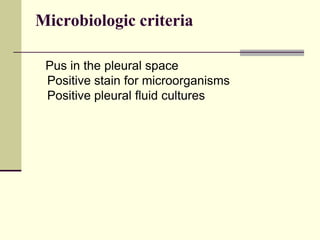
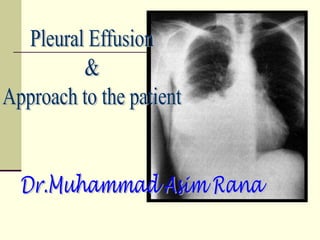
Pleural effusion is the accumulation of fluid in the pleural space, resulting from either excess fluid production or decreased absorption. There are two types: transudative, caused by systemic factors, and exudative, due to local pathological processes. Diagnosis involves clinical assessment, imaging techniques, and pleural fluid analysis to determine the underlying cause for appropriate treatment.




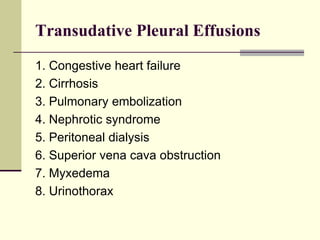
![Transudative pleural effusions
result from alteration of hydrostatic and
oncotic factors that increase the formation or
decrease the absorption of pleural fluid (e.g.,
increased mean capillary pressure [heart
failure] or decreased oncotic pressure
[cirrhosis or nephrotic syndrome]).](https://image.slidesharecdn.com/approachtopleuraleffusion-140205151929-phpapp01/85/Approach-to-pleural-effusion-6-320.jpg)