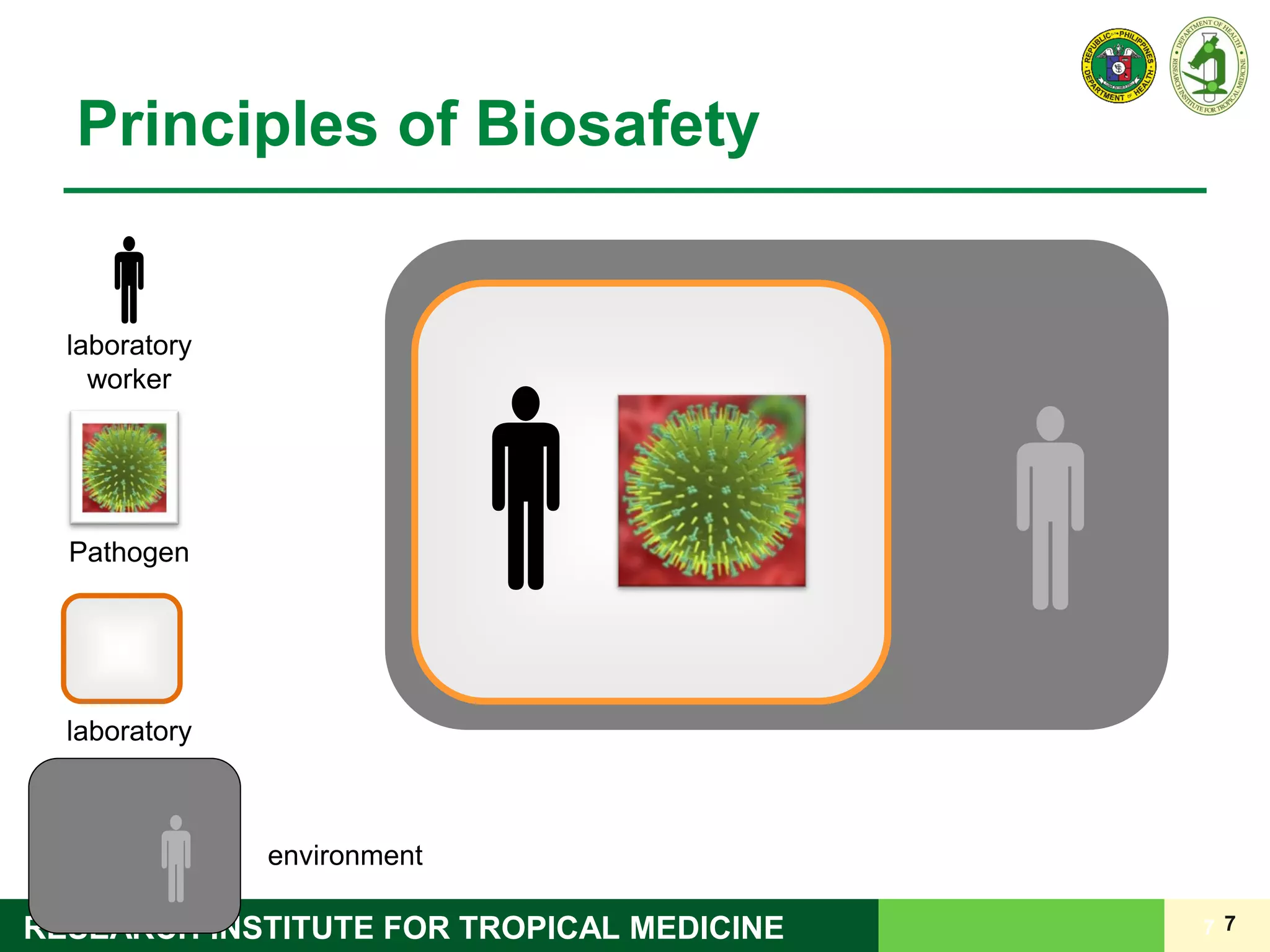
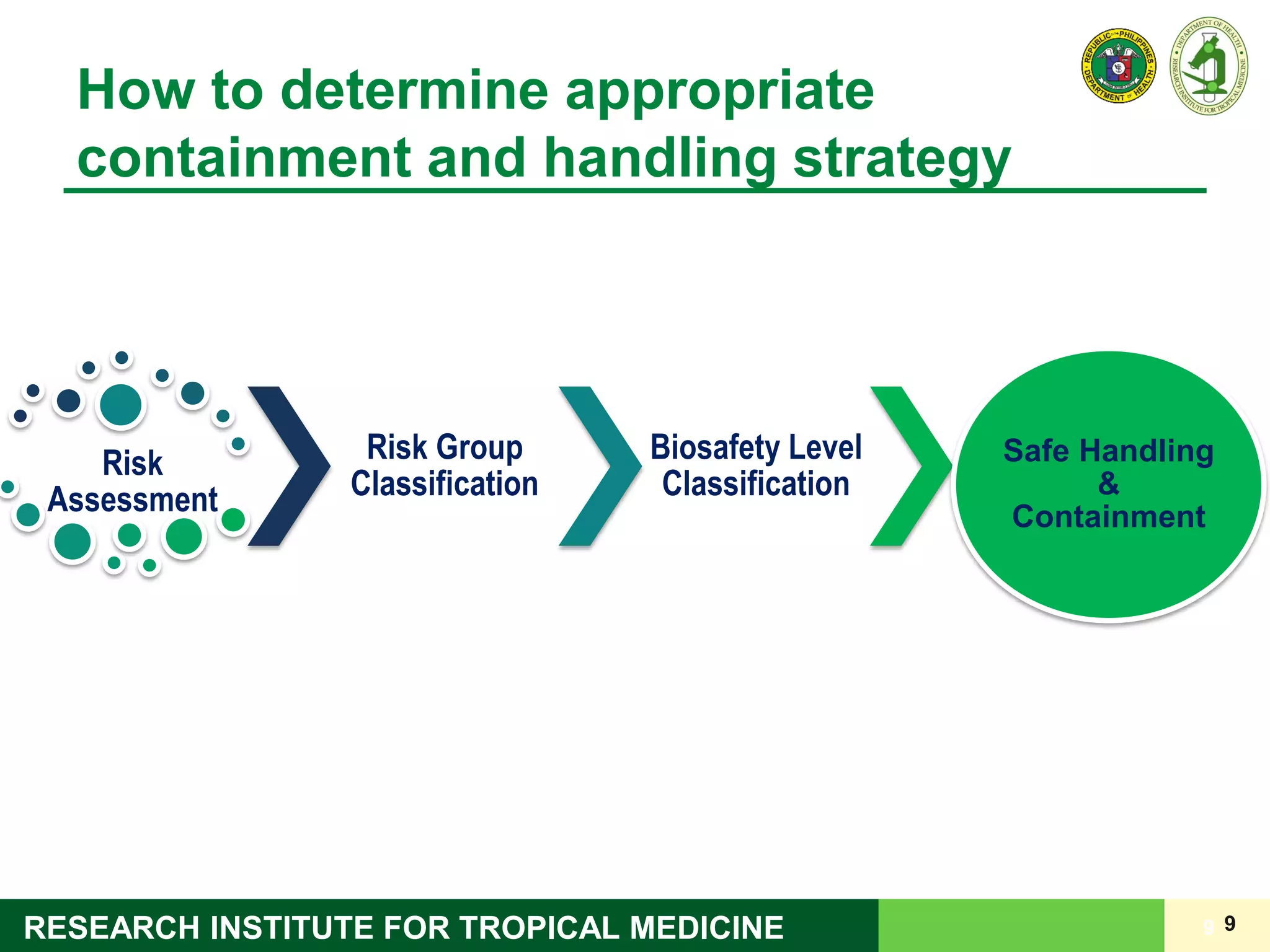
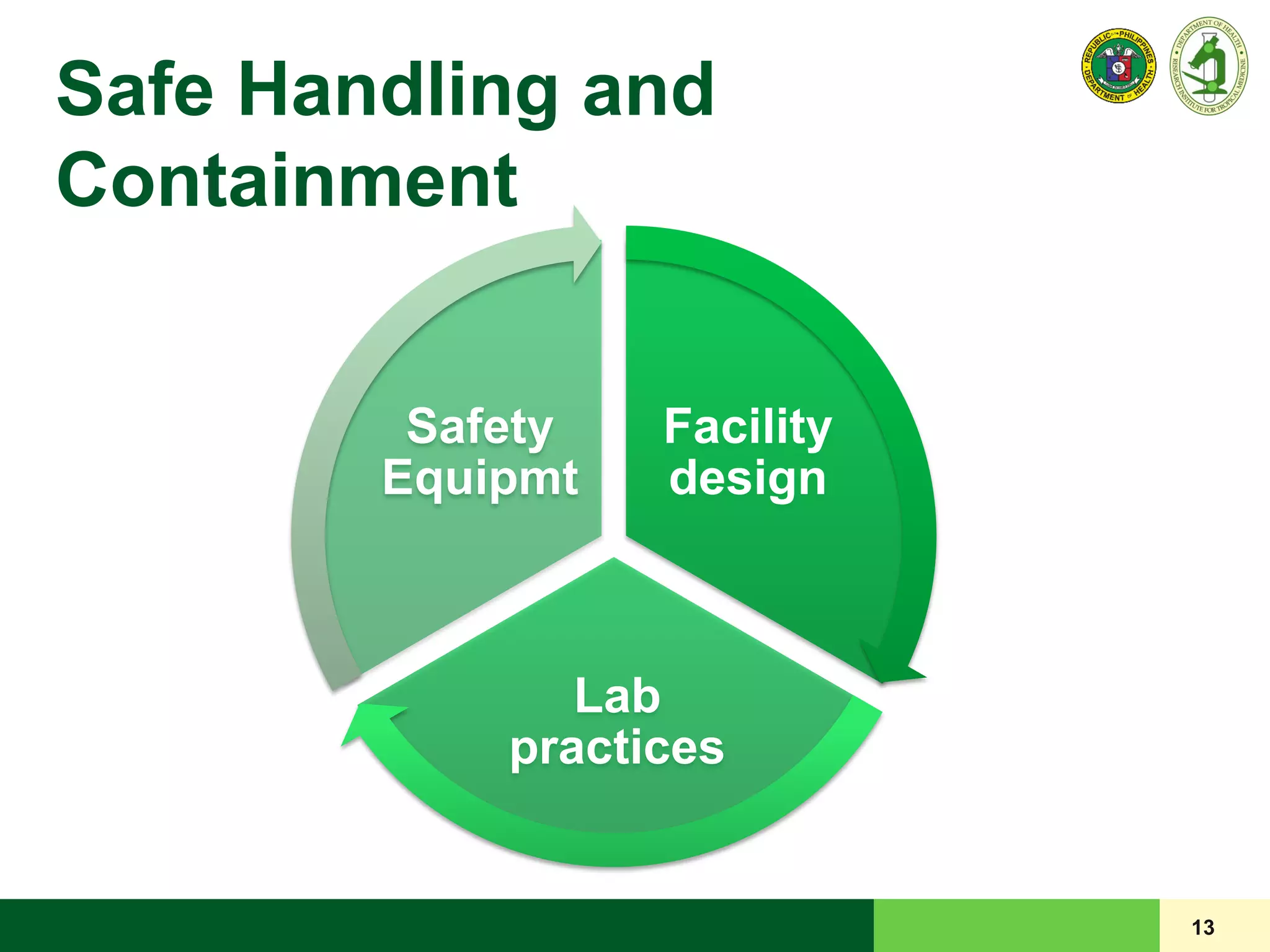
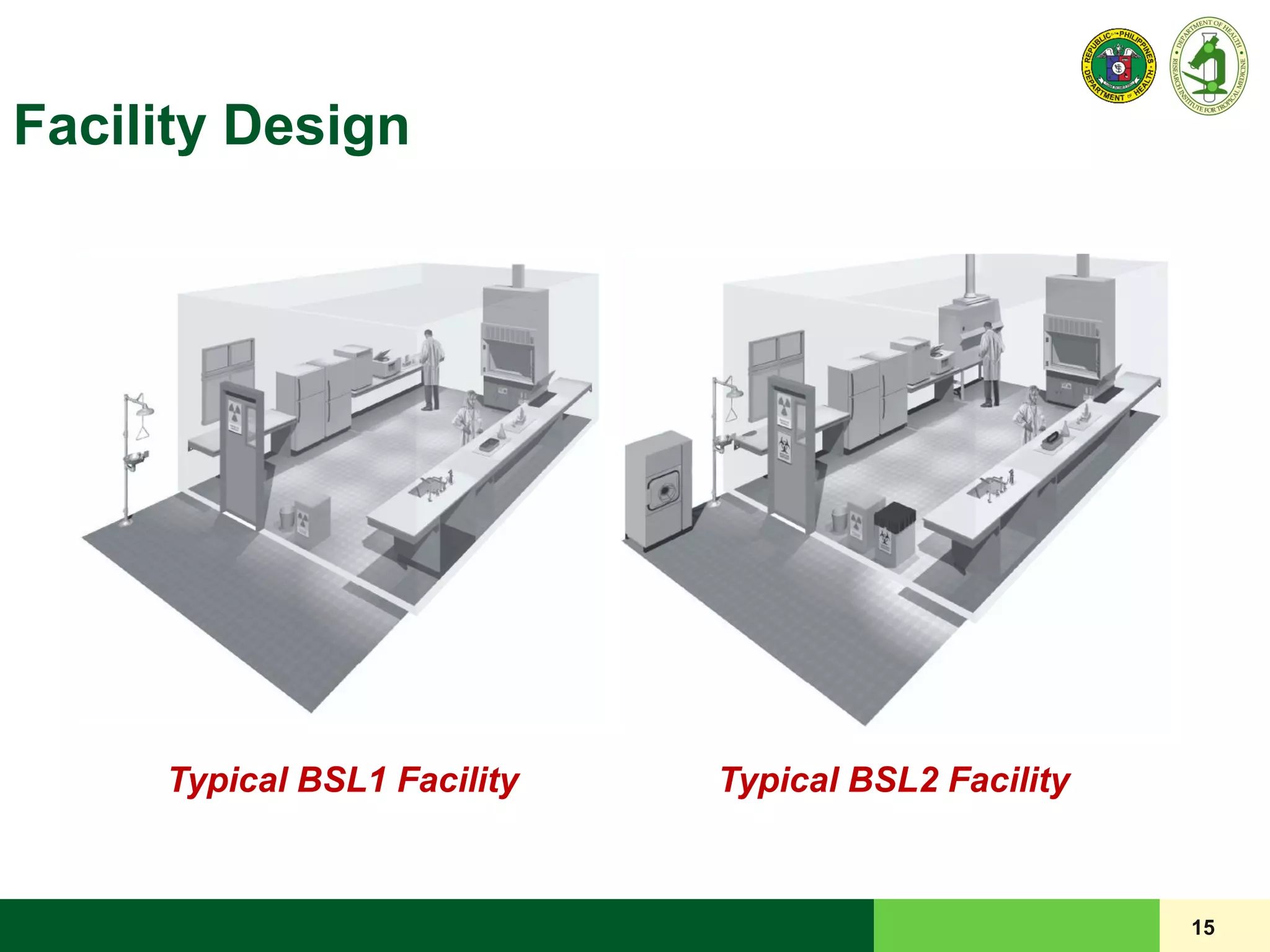
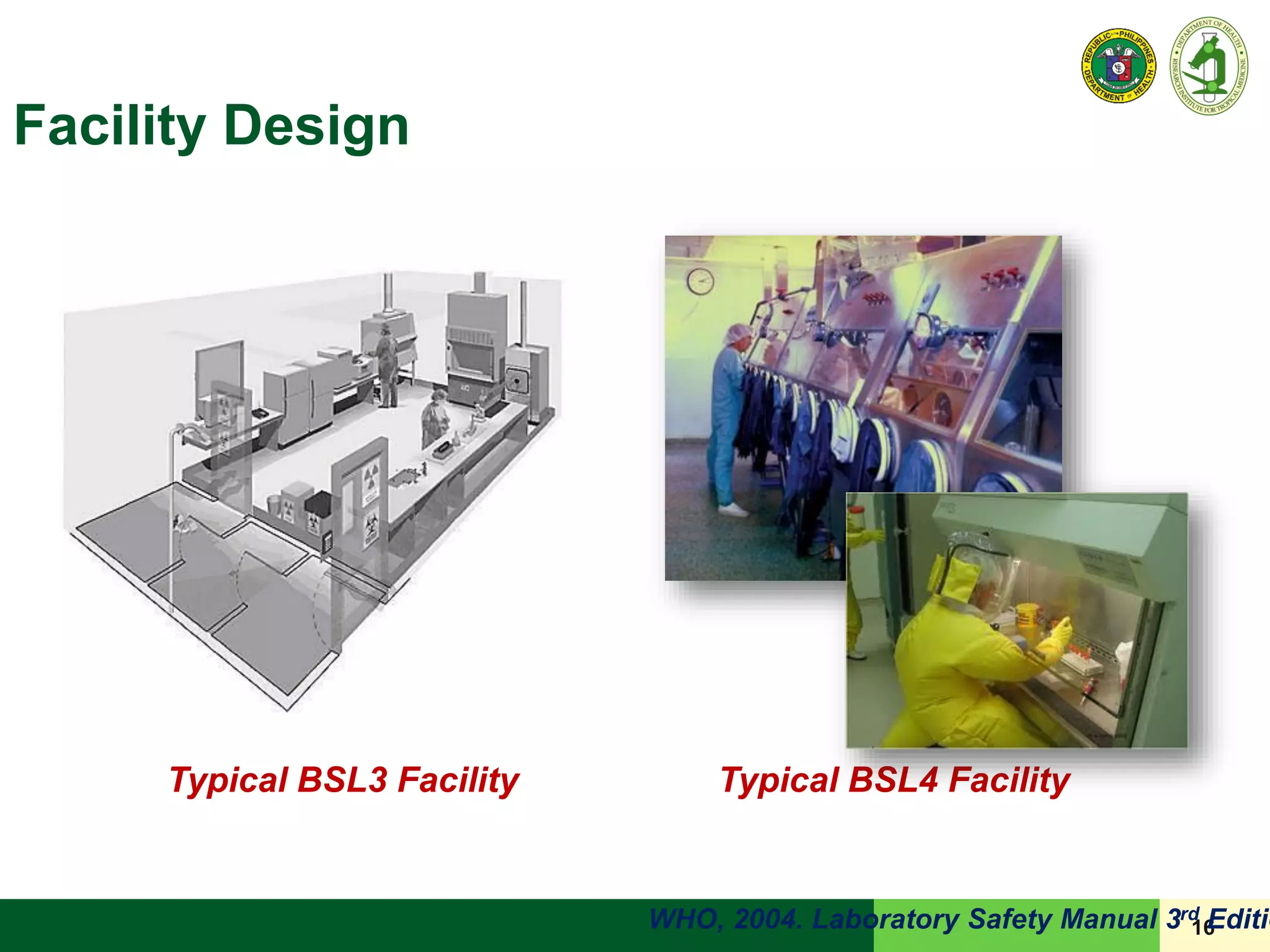
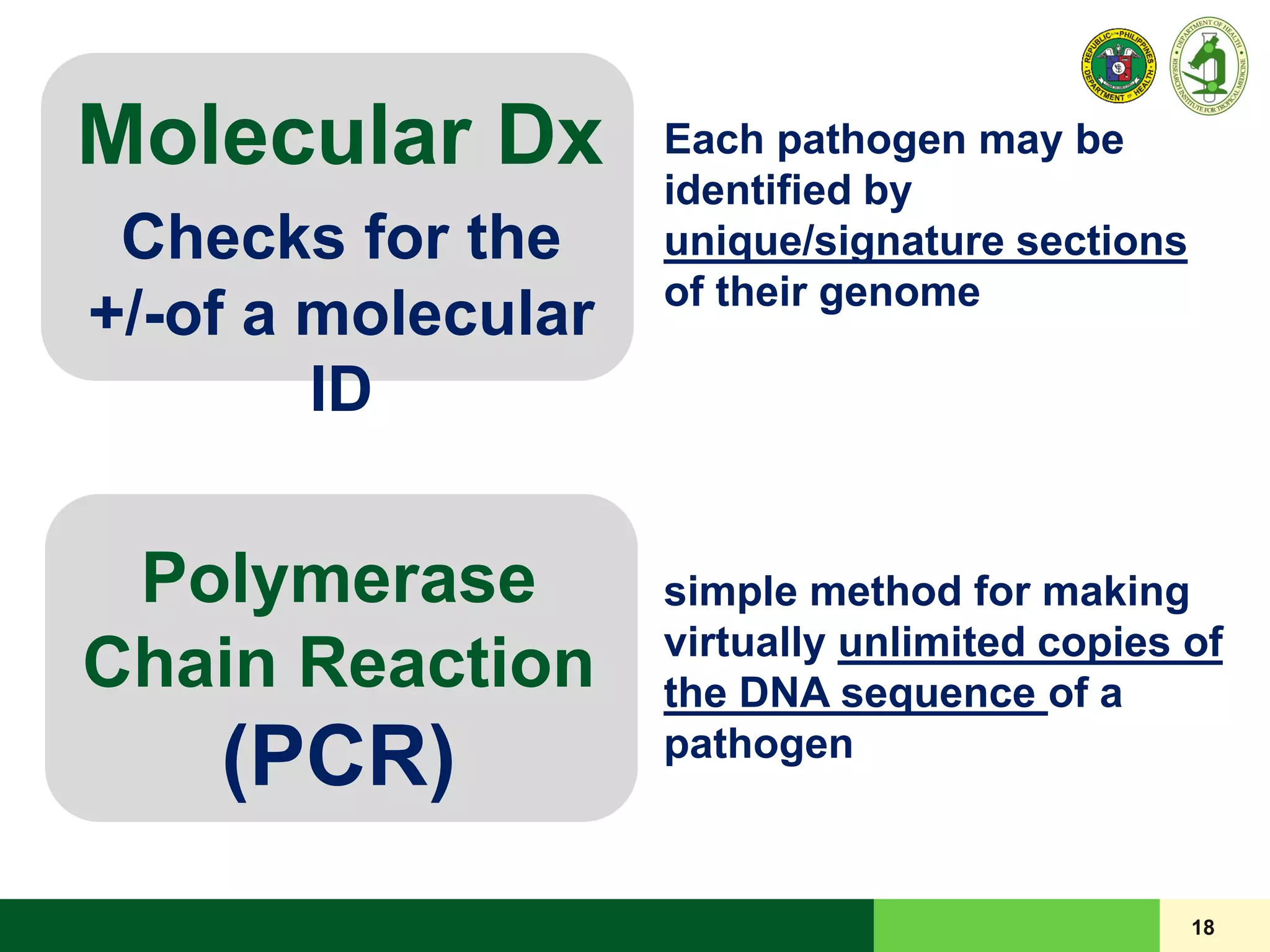
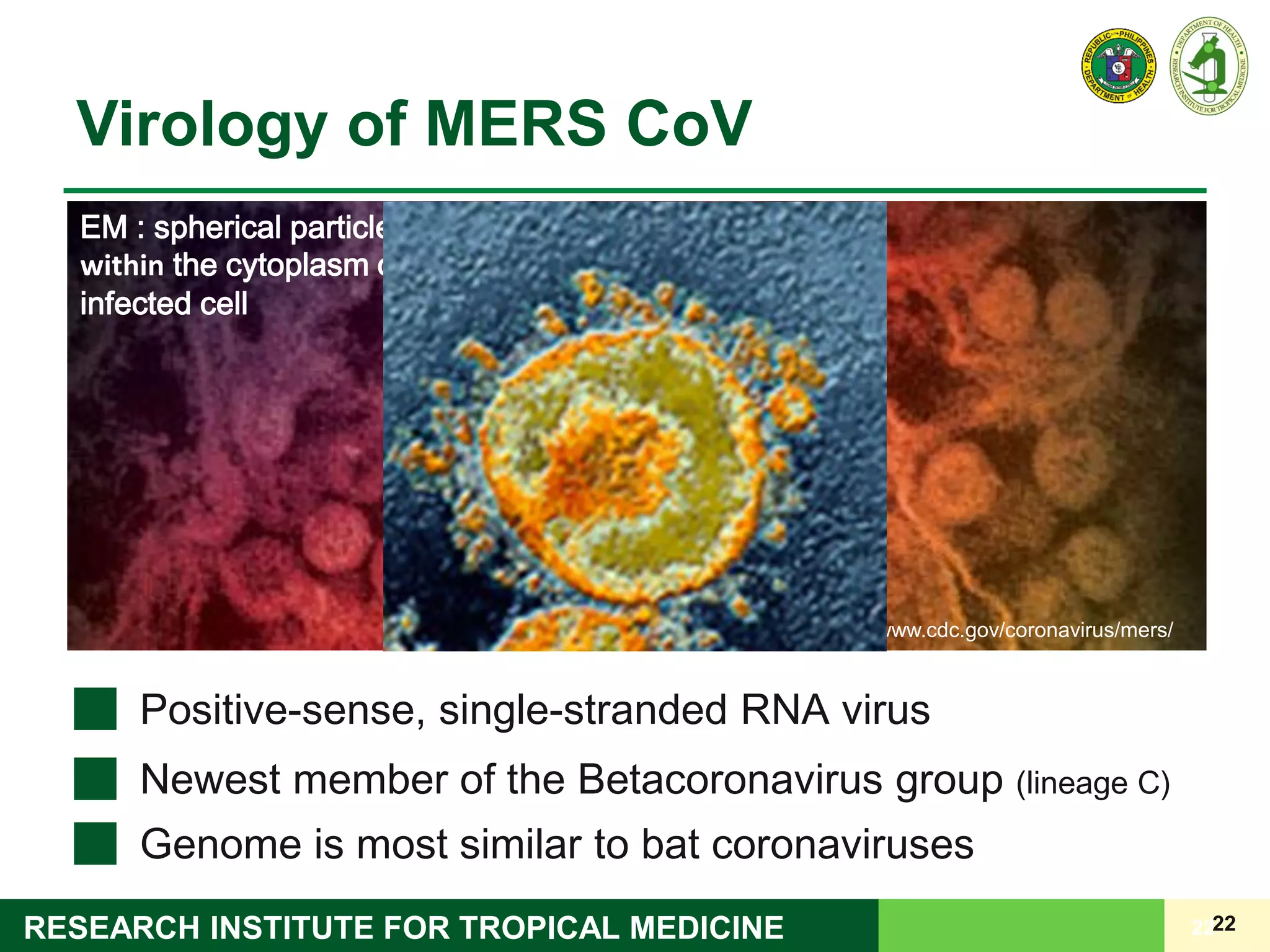
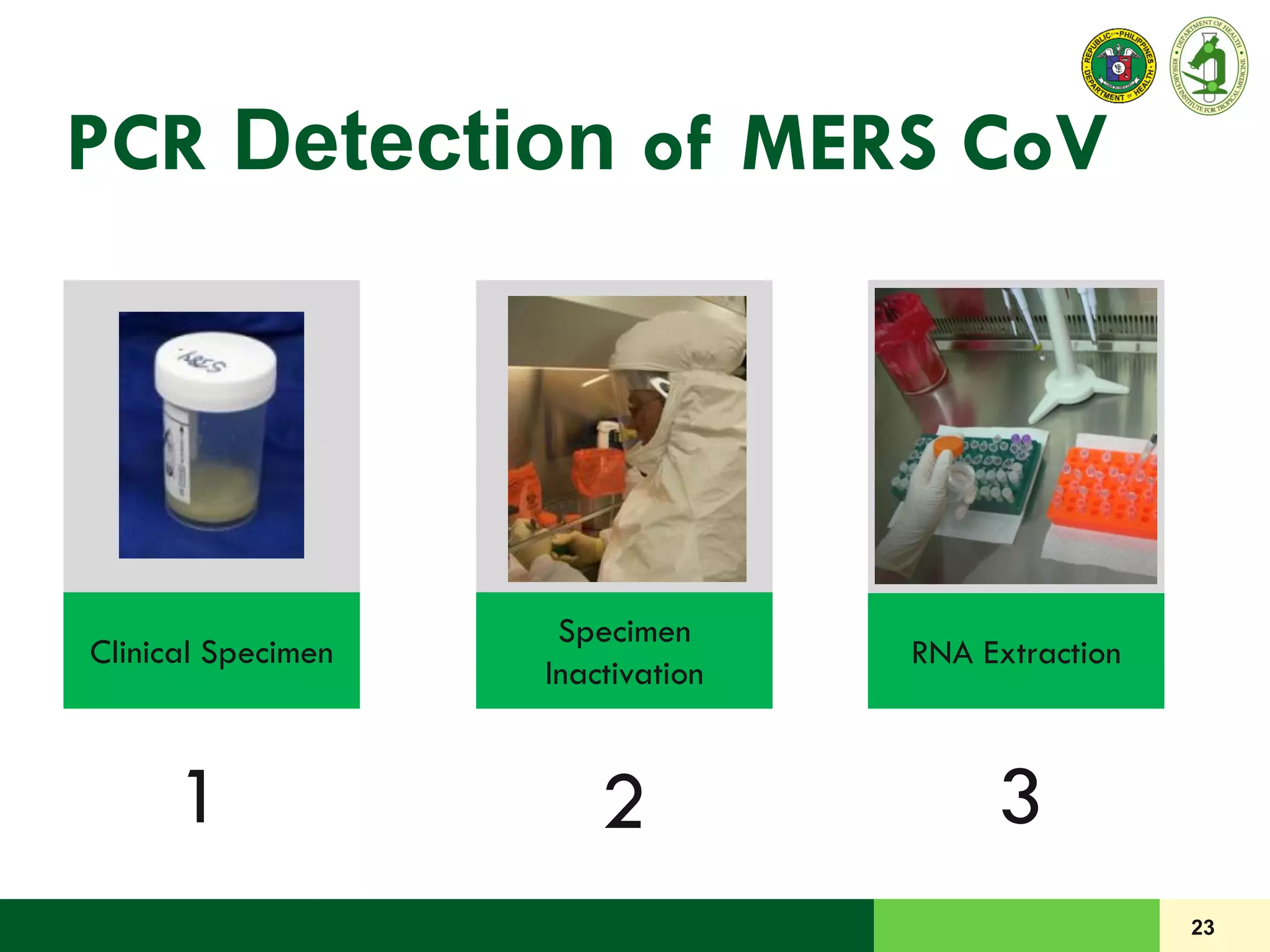
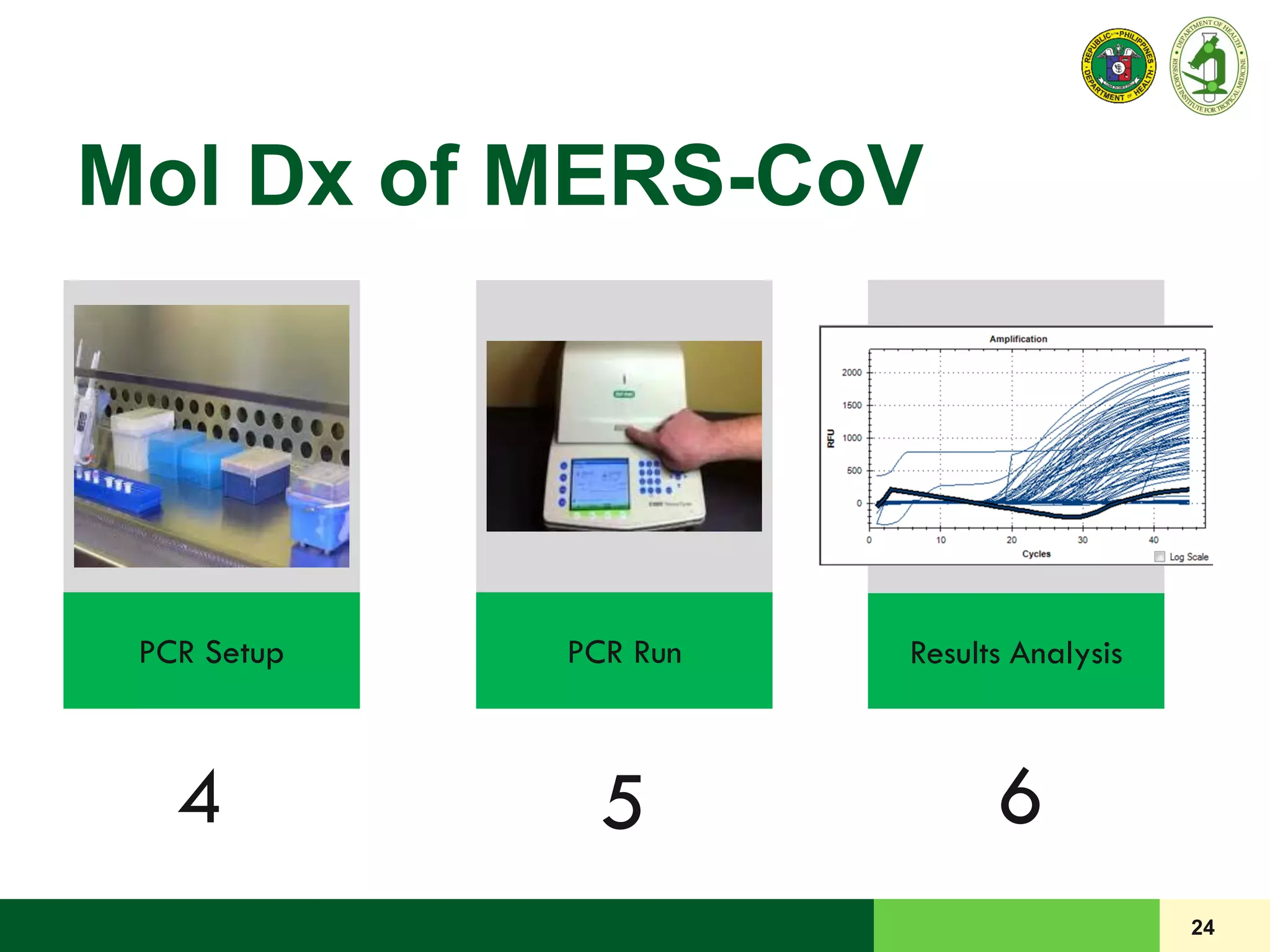
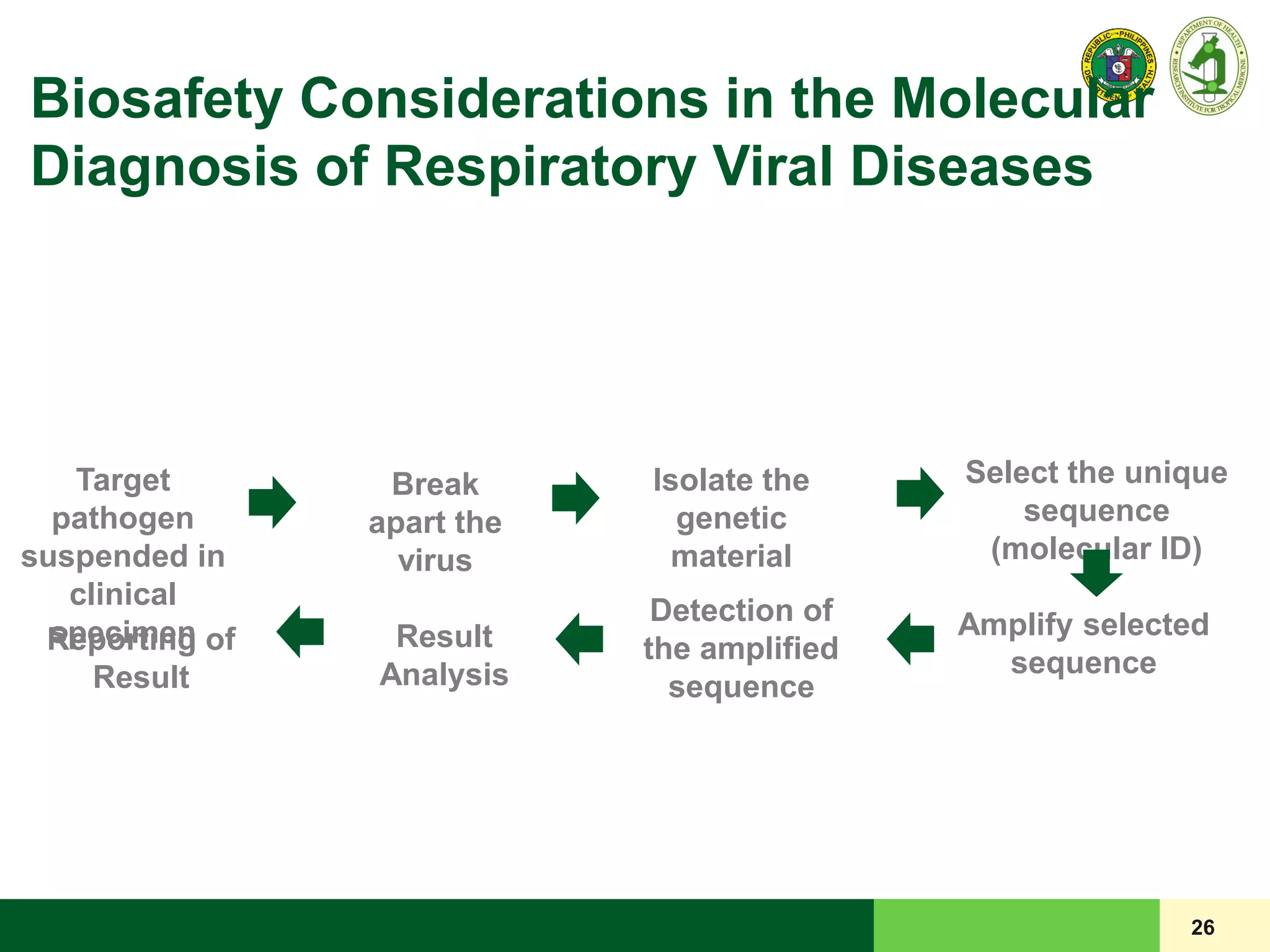
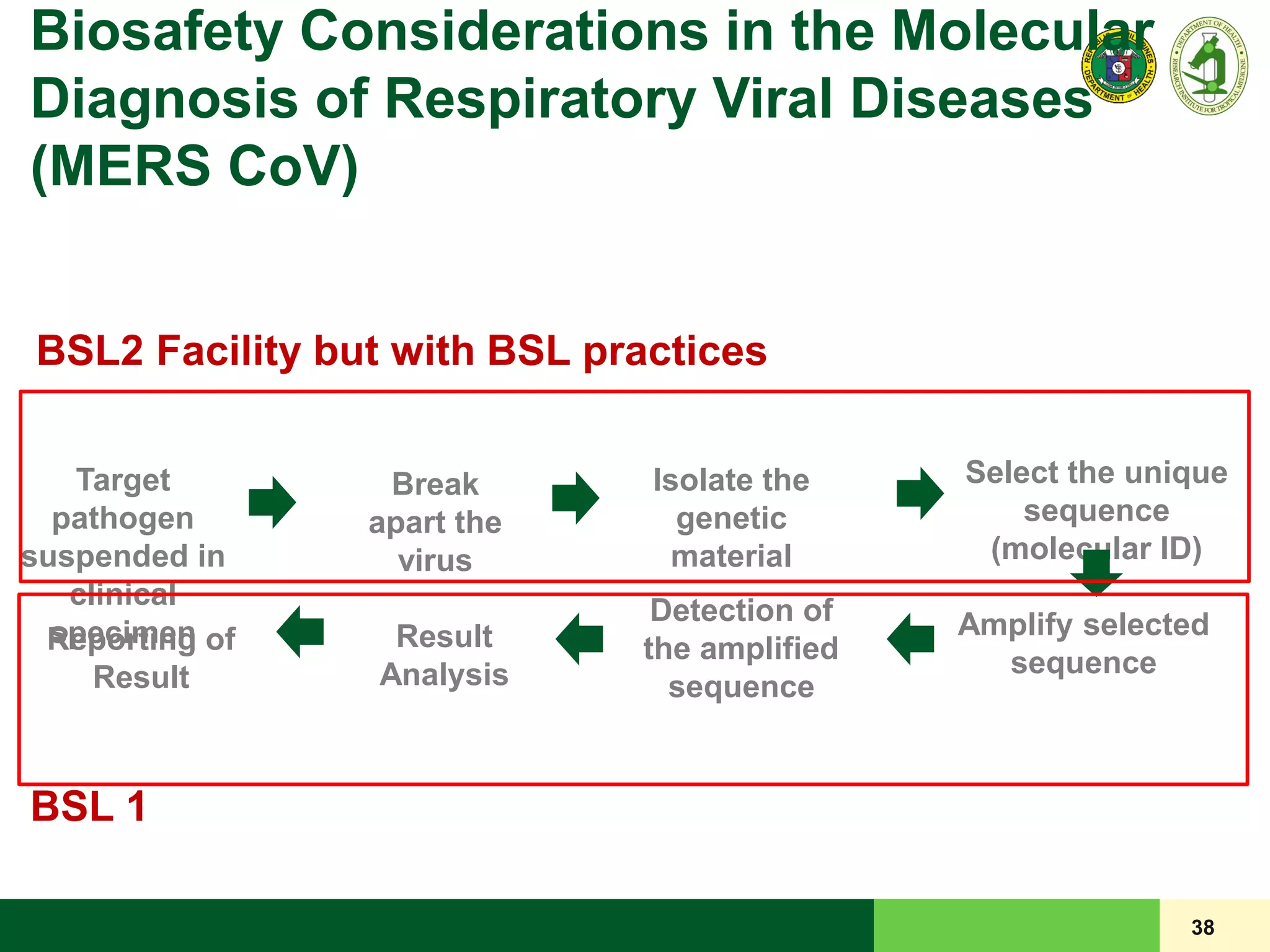
This document discusses laboratory biosafety in the molecular diagnosis of emerging respiratory infectious diseases. It covers the changing landscape of infectious diseases, PCR-based detection of pathogens like MERS-CoV, biosafety principles, risk assessment, containment levels, and safe handling practices. Specific considerations are given to conducting molecular detection of MERS-CoV, which is a biosafety level 3 pathogen, in a way that protects laboratory workers, the community, and the environment.























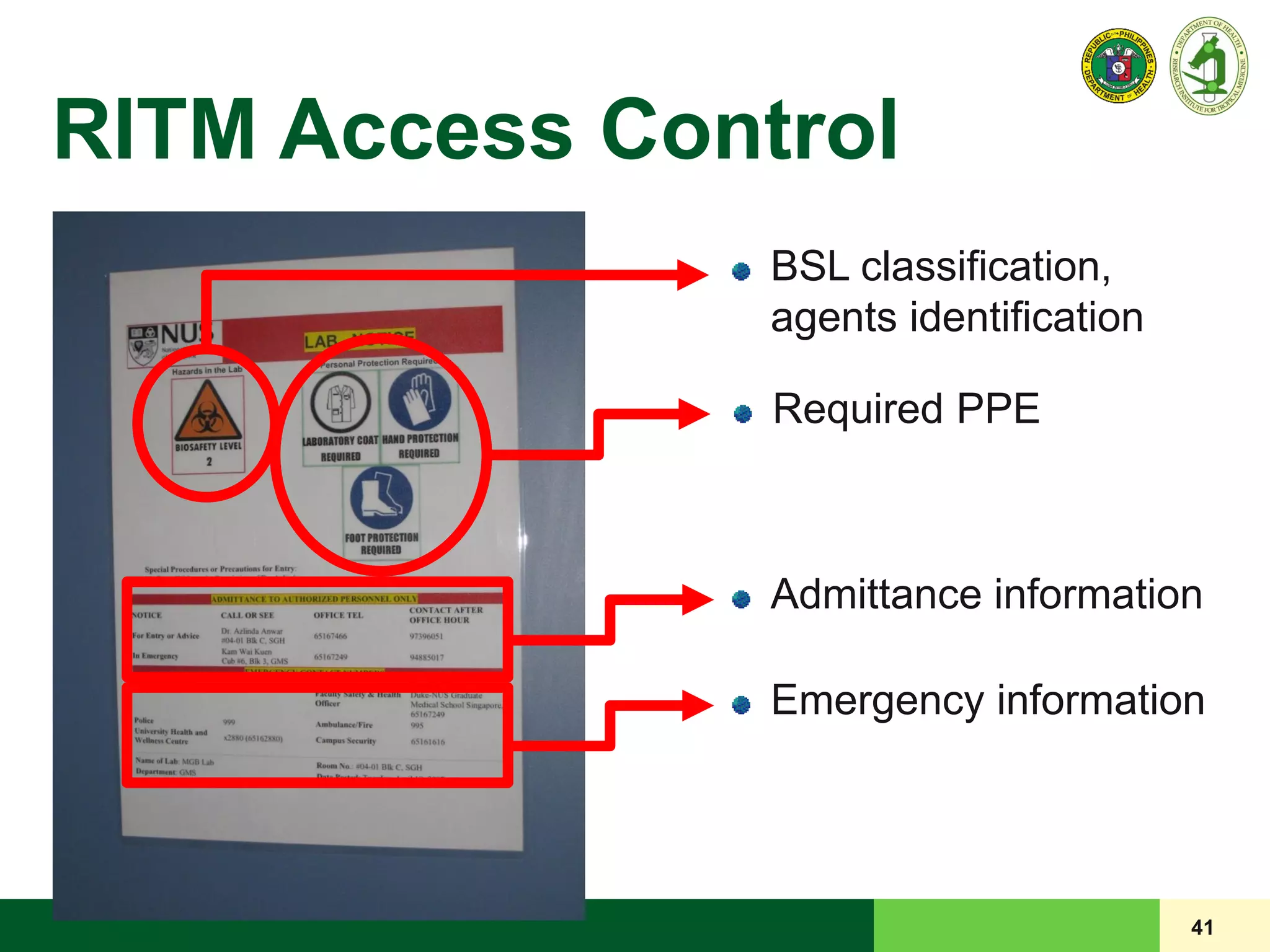
![40
Door to the laboratory must be closed,
[preferably] with self-closing mechanism
Appropriate warning sign where infectious
microorganisms are handled
LRD POLICY
LRD Standard Access Control Information and
Biohazard Warning (if applicable) signage for each
laboratory
RITM Access Control](https://image.slidesharecdn.com/laboratorybiosafety-160308035302/75/Laboratory-Biosafety-40-2048.jpg)