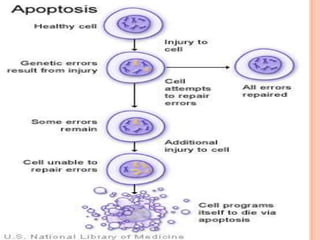
This document summarizes apoptosis, or programmed cell death. It describes how apoptosis involves characteristic cellular changes, including blebbing and nuclear fragmentation. Between 50-70 billion cells die daily in the human body through apoptosis. The process was first described in 1842 and the term "apoptosis" was introduced in 1972. Apoptosis occurs through intrinsic and extrinsic pathways, and involves caspase proteins. It plays an important role in development, the immune system, and maintaining tissue homeostasis.