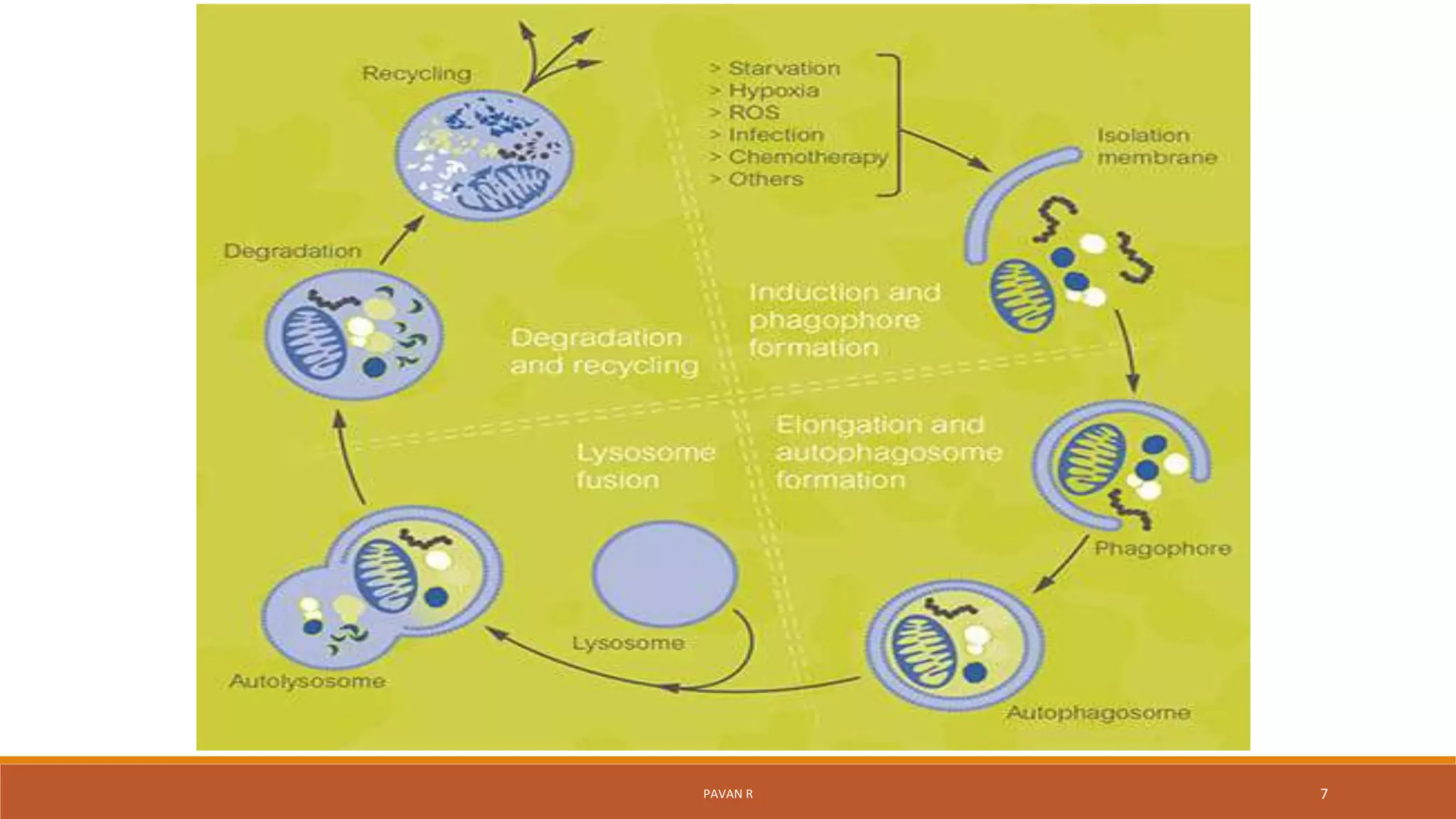
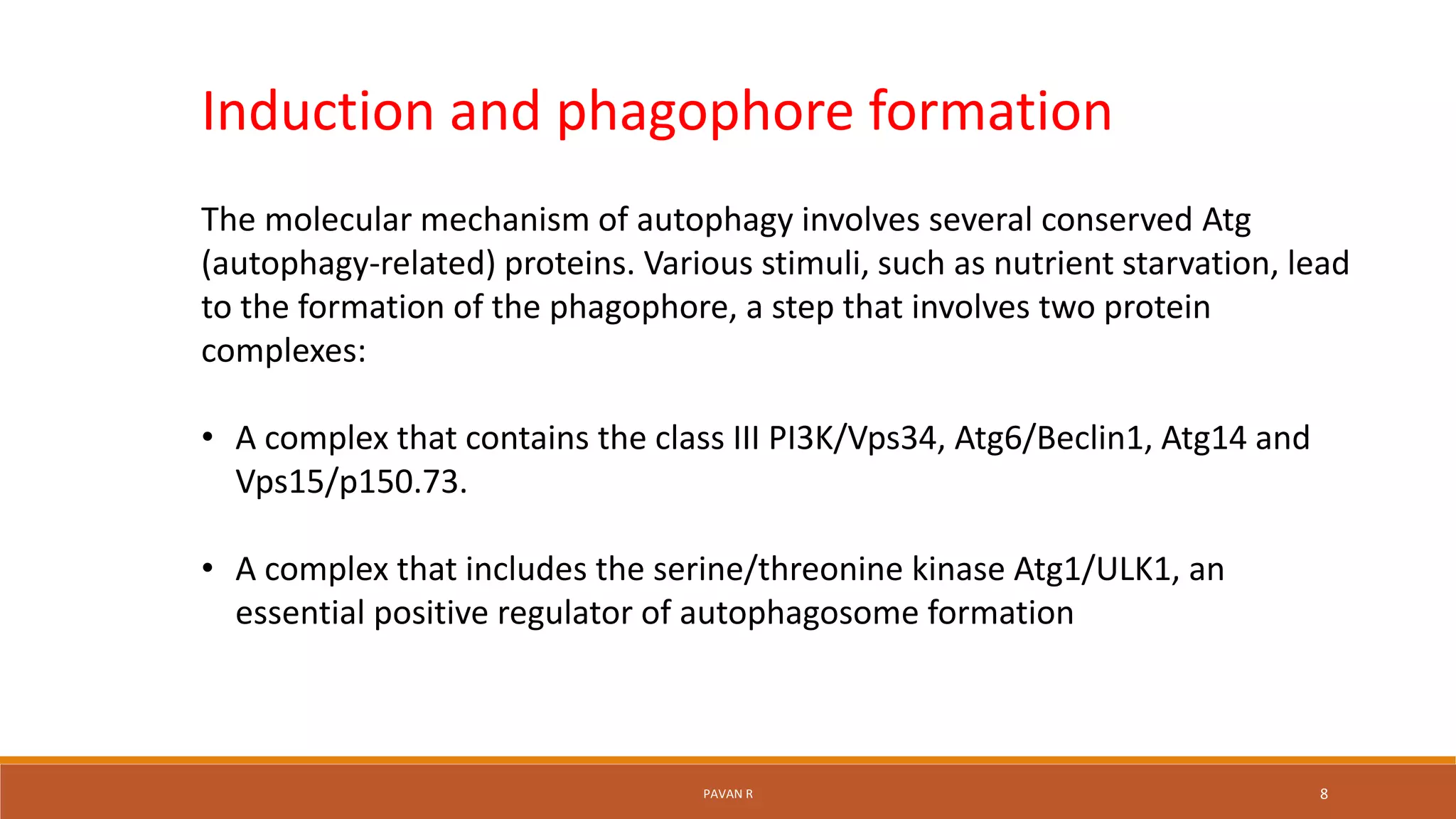
The document discusses autophagy, which is a normal physiological process in the body that deals with the destruction and recycling of cellular components. It was first observed in 1962 and involves several conserved autophagy-related proteins and multiple stages. There are three main types of autophagy: microautophagy, macroautophagy, and chaperone-mediated autophagy. Microautophagy involves direct engulfment into lysosomes, macroautophagy uses double-membrane vesicles called autophagosomes, and chaperone-mediated autophagy selectively transports proteins across lysosome membranes. Autophagy is regulated by various factors and plays important roles in cellular