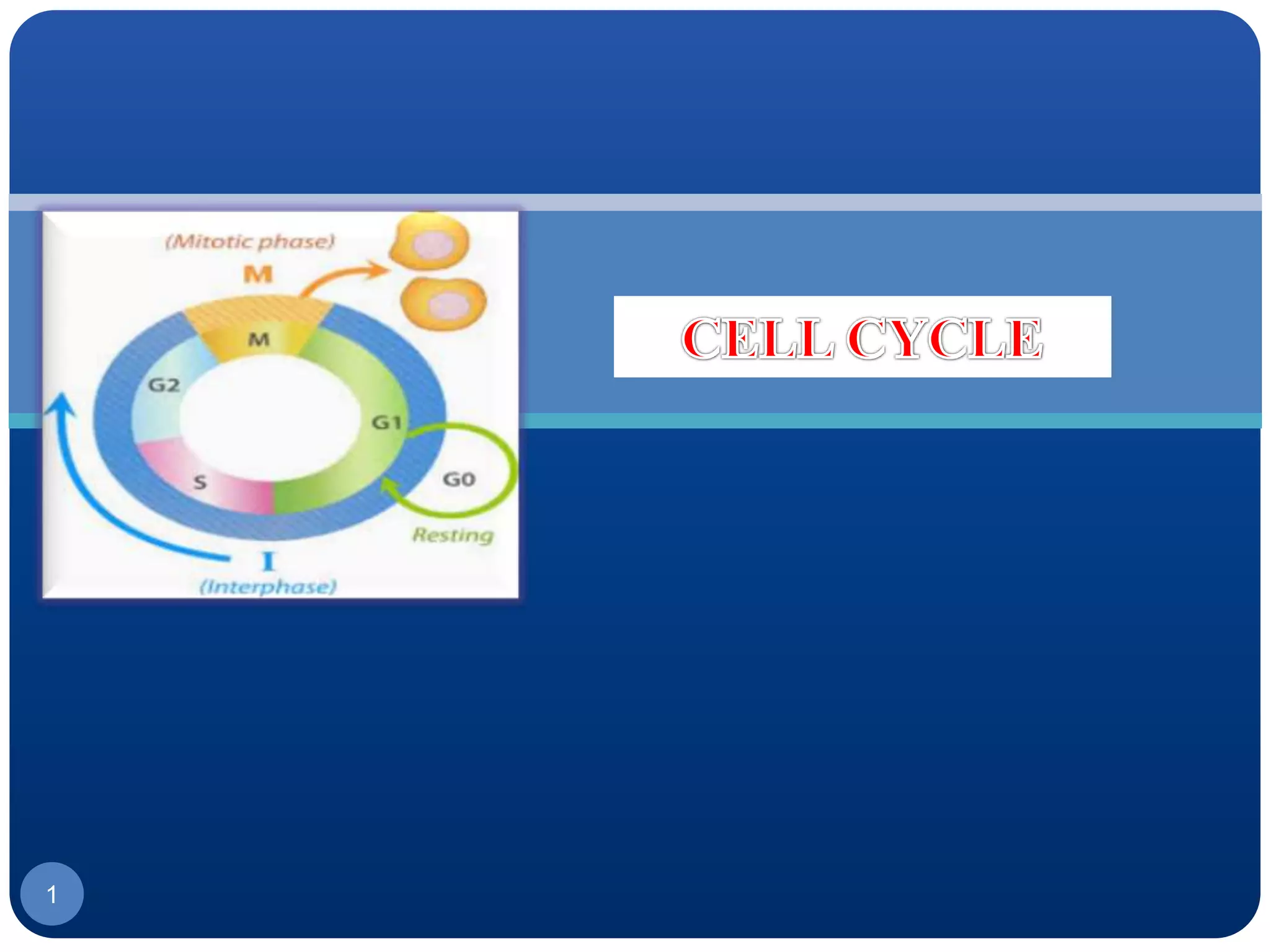
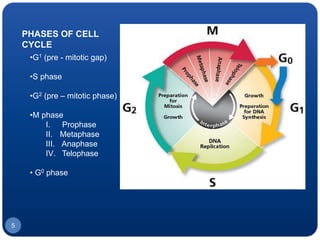
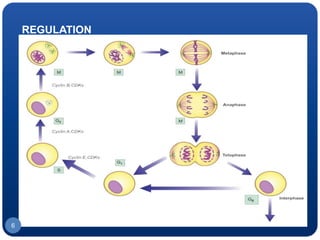
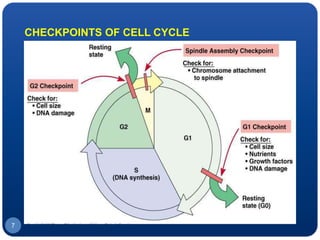
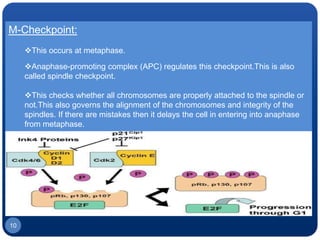
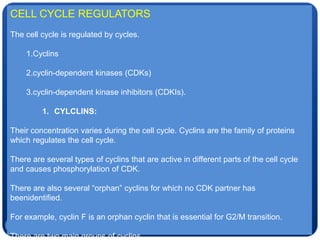
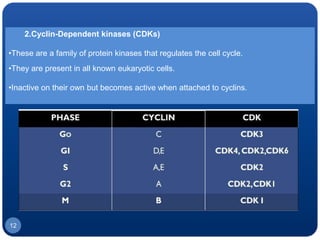
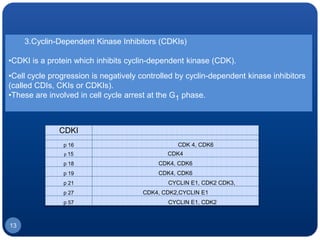
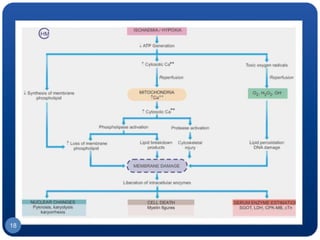
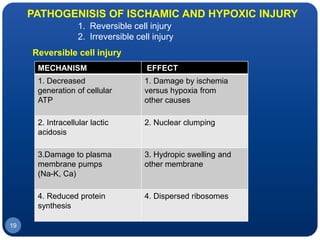
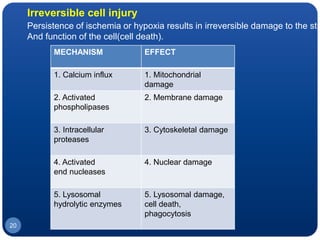
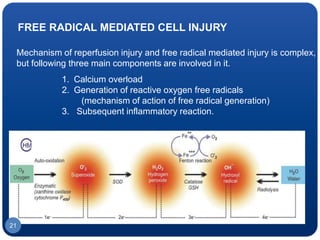
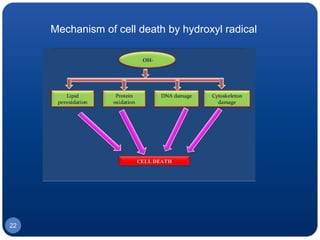
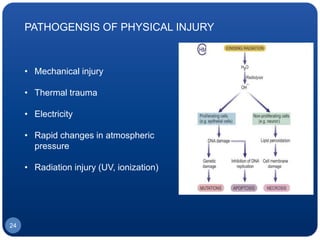
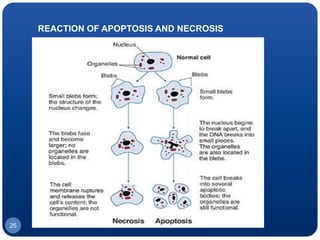
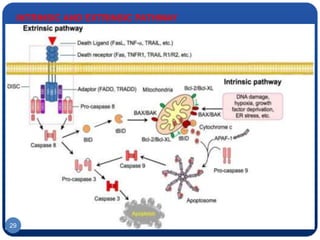
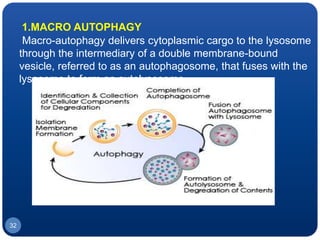
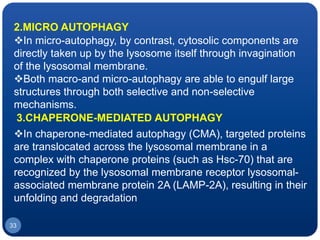
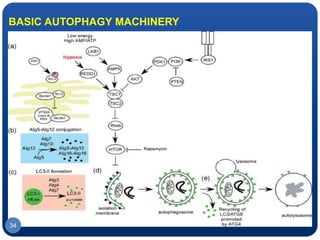
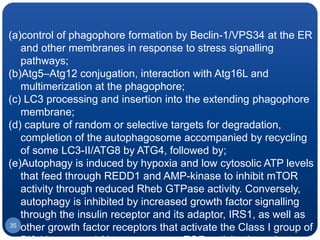
The document discusses cell cycle regulation and cell injury. It describes the phases of the cell cycle including G1, S, G2, and M phases. Checkpoints at G1 and G2 regulate progression through the cycle. The cycle is controlled by cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors. Cell injury can be caused by genetic or acquired factors like ischemia, toxins, radiation. Injury may lead to reversible or irreversible damage through effects on mitochondria, membranes, and DNA. Cells undergo programmed cell death through necrosis, apoptosis, or autophagy in response to injury.