1) Anthrax is caused by the bacterium Bacillus anthracis. It can cause serious illness in humans and animals.
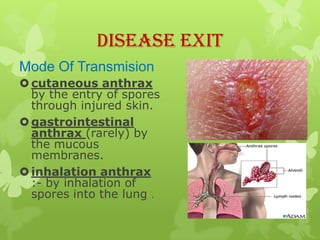
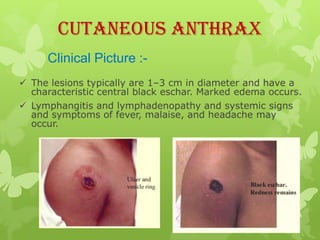
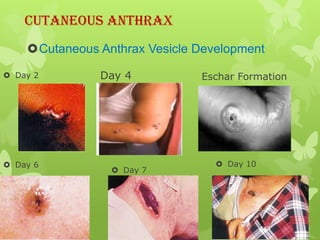
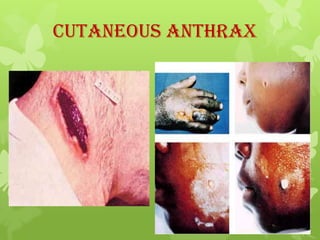
2) There are three main types of anthrax disease in humans - cutaneous, inhalation, and gastrointestinal. Cutaneous anthrax is the most common, usually occurring after exposure to infected animals or contaminated products.
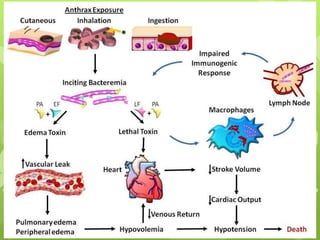
3) Anthrax bacteria produce toxins that are major virulence factors. The anthrax toxins are composed of three proteins that combine to cause tissue damage and edema.
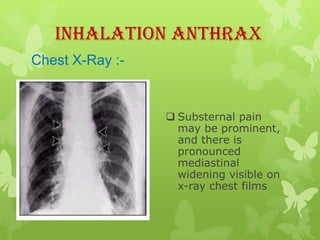
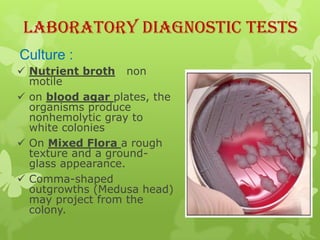
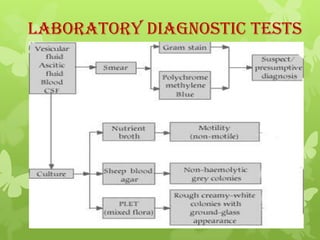
4) Diagnosis involves lab tests of samples from lesions, blood, or sputum to identify B. anthracis. Treatment involves antibiotics such as