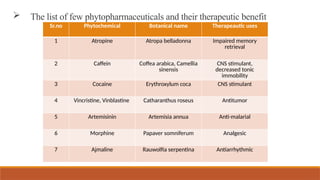
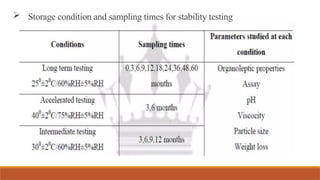
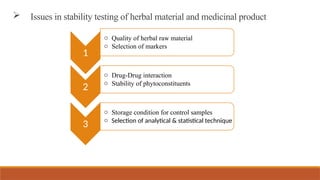
This document provides an overview of stability testing for phytopharmaceuticals, outlining the regulatory requirements and protocols essential for drug stability assessment. It defines phytopharmaceuticals and discusses their historical significance, regulatory framework, and the importance of stability testing in maintaining the quality of herbal medicines. Additionally, it highlights the challenges in stability testing, including the selection of appropriate analytical techniques and testing parameters for ensuring the safety and efficacy of herbal products.