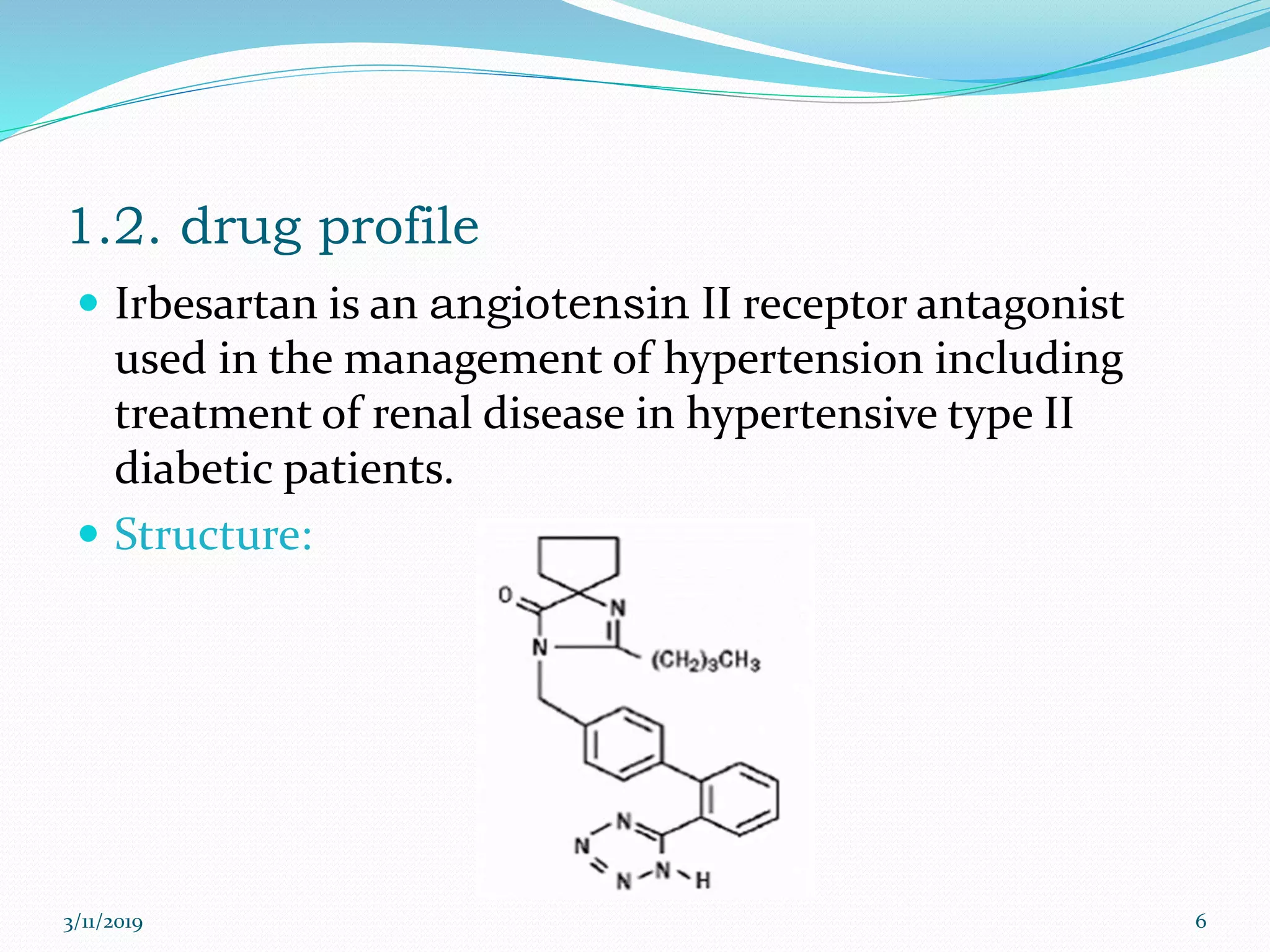
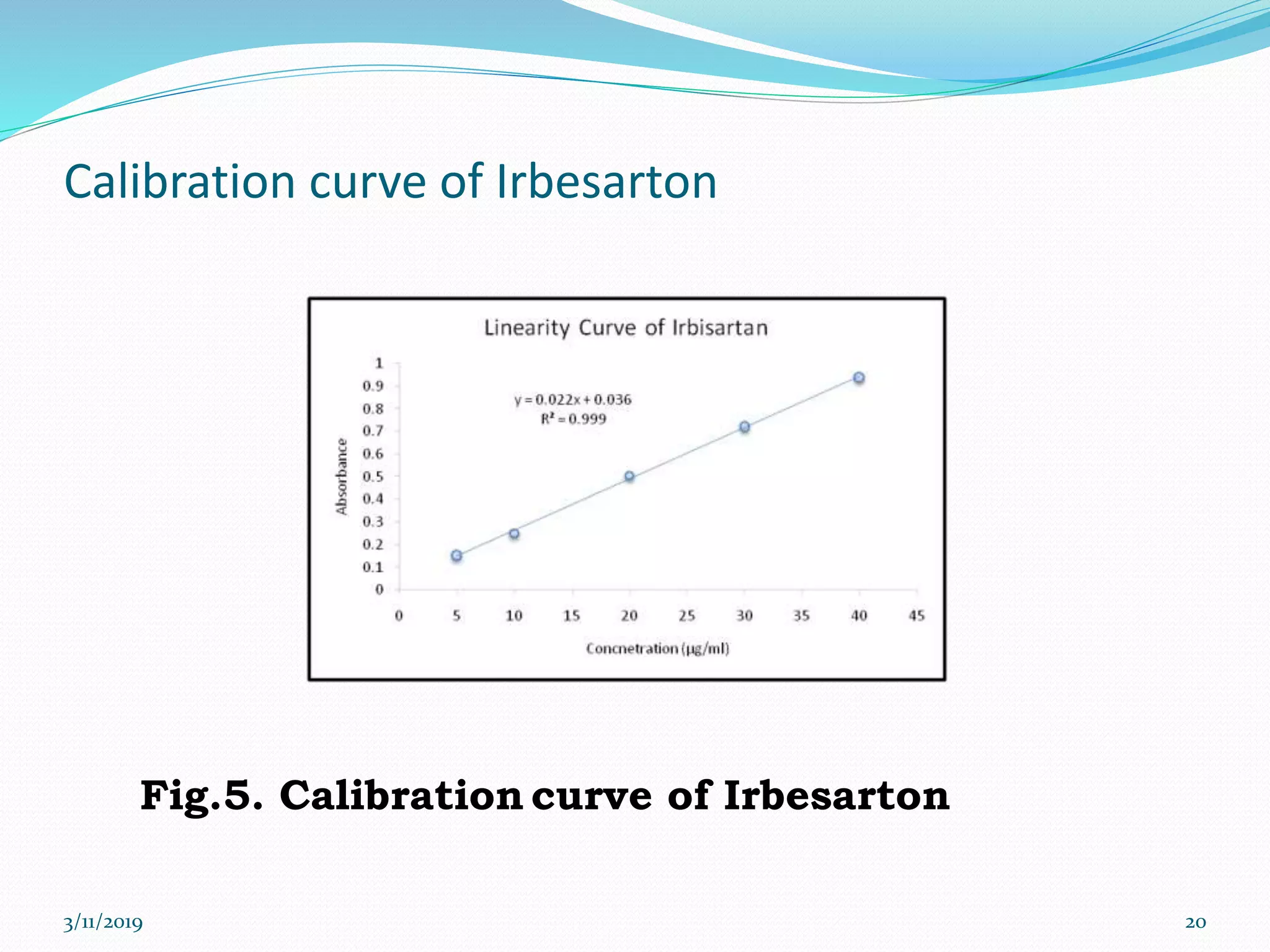
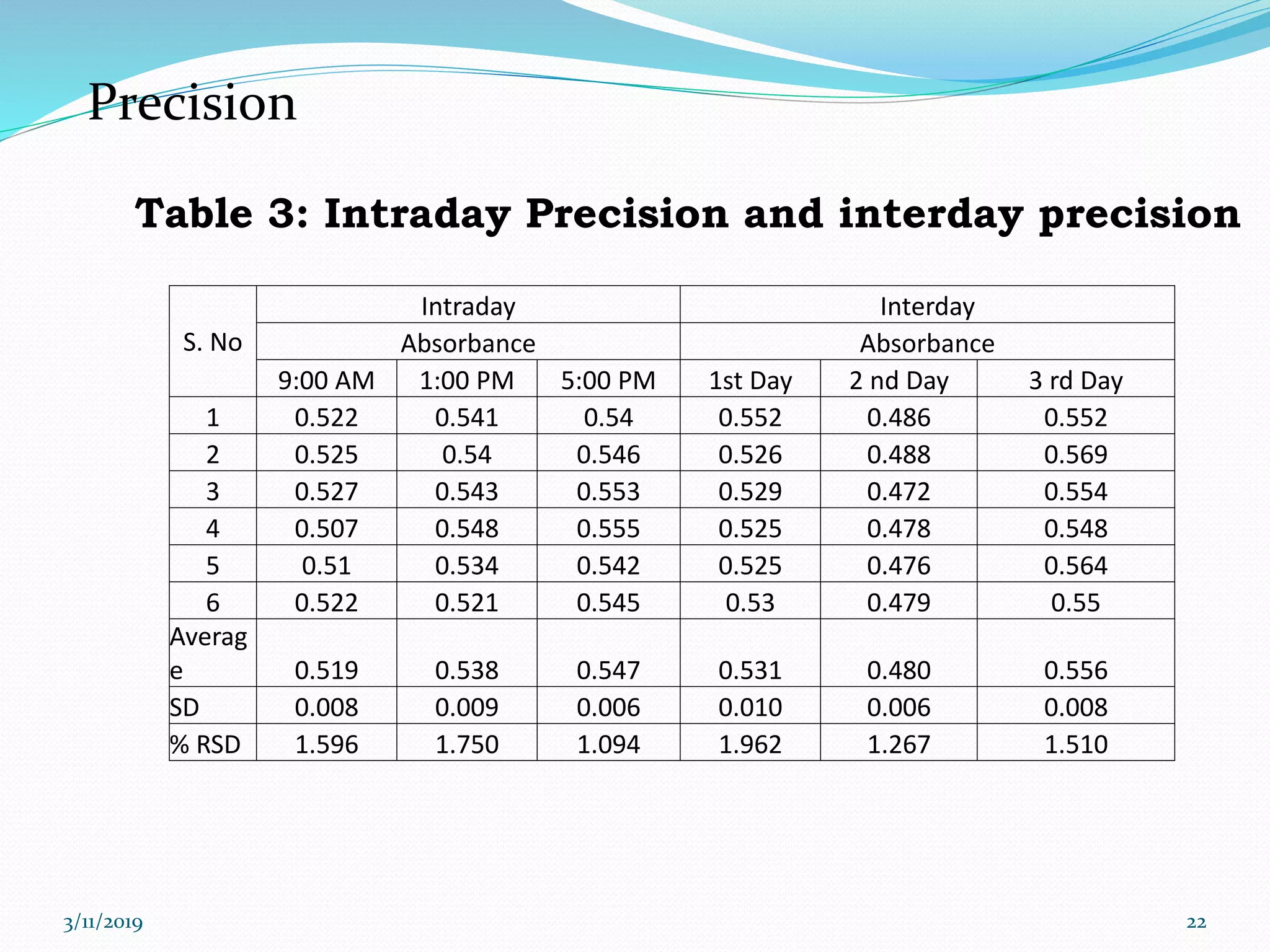
This document presents the development and validation of a UV spectrophotometric method for the analysis of Irbesartan in tablet formulations. The method involves preparing standard and sample solutions of Irbesartan in 0.1N NaOH, scanning them from 200-400nm to obtain spectra, and measuring absorbance of the sample at 255nm. The method is linear from 5-40μg/ml. Accuracy, precision, specificity, robustness and degradation studies establish that the method is valid for analysis of Irbesartan in tablets.