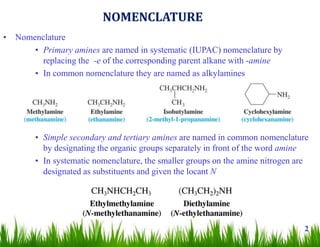
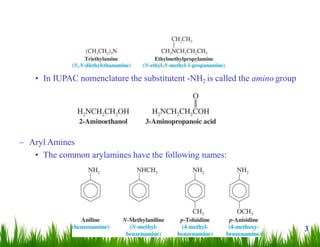
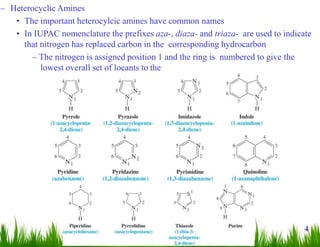
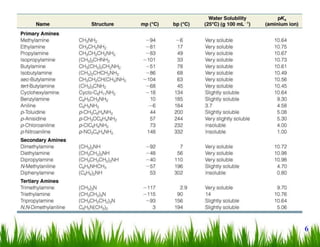
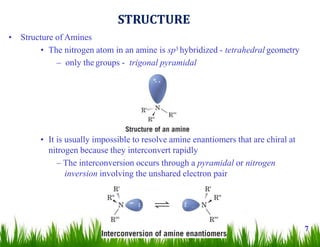
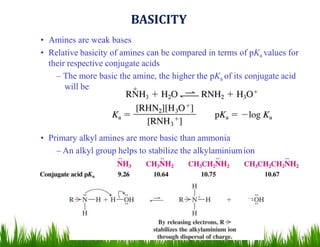
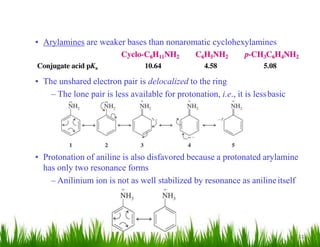
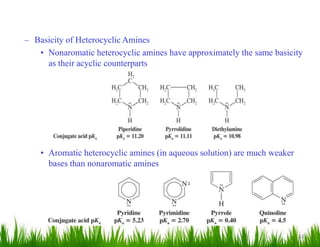
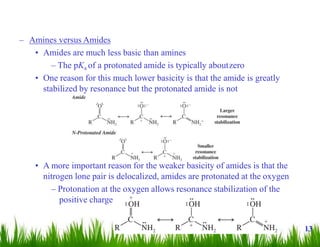
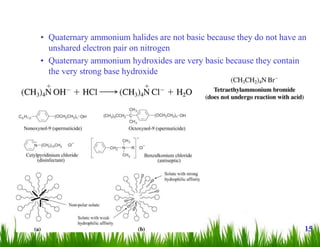
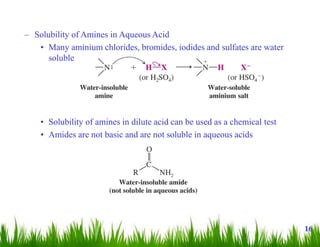
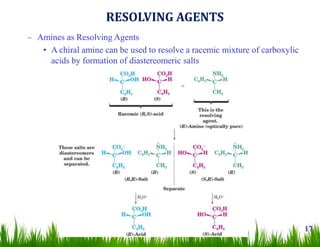
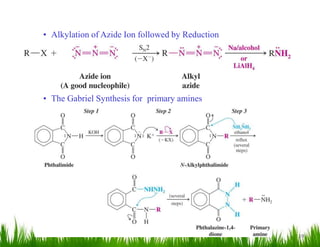
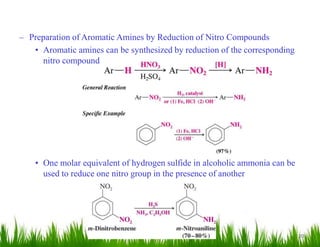
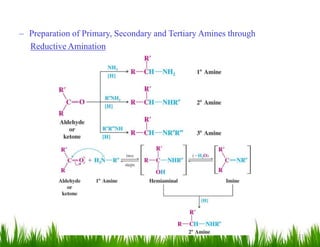
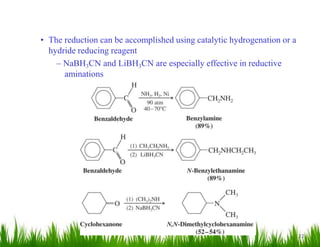
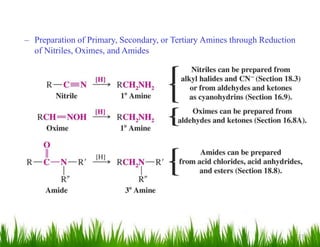
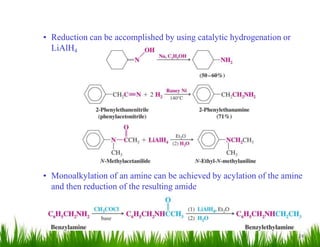
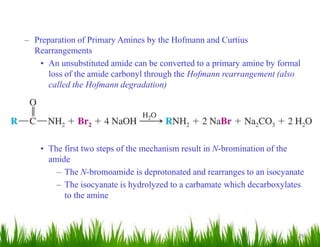
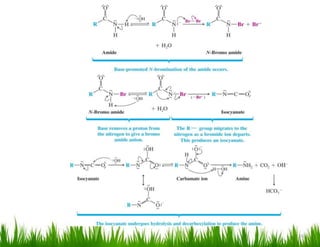
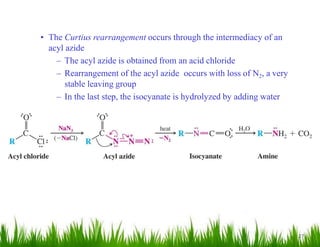
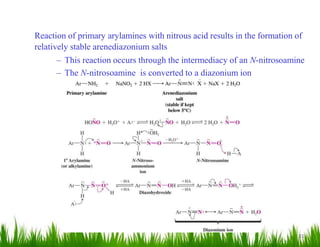
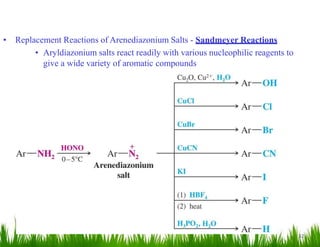
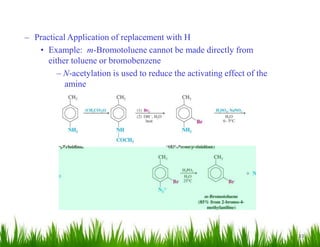
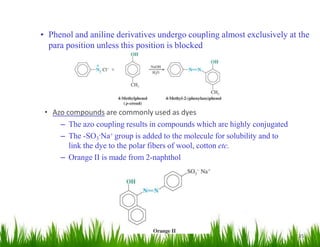
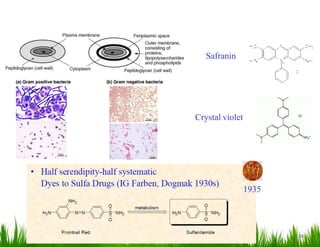
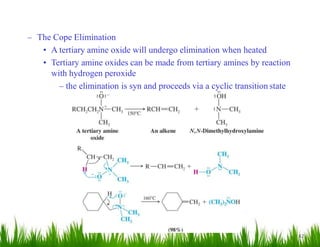
1. The document discusses the nomenclature, physical properties, structure, basicity, and reactions of primary, secondary, and tertiary amines. It covers topics such as IUPAC nomenclature, hydrogen bonding abilities, inversion of chiral amines, relative basicities, and reactions including oxidation, diazotization, and coupling reactions.
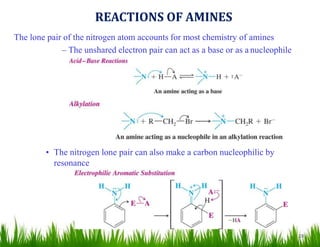
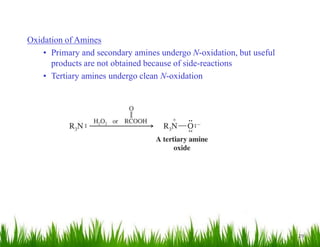
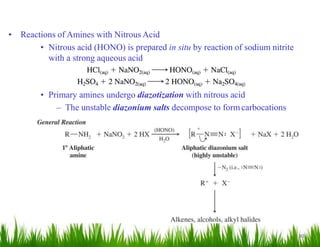
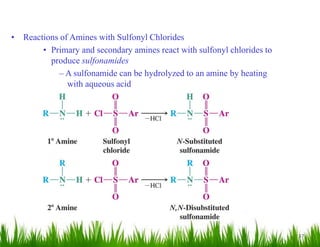
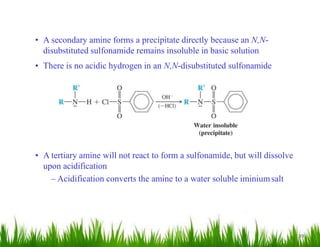
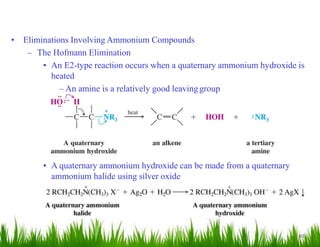
2. Amines can undergo a variety of reactions due to the lone pair on the nitrogen atom, including acting as a base or nucleophile. Reactions covered include oxidation, diazotization with nitrous acid, coupling reactions, and eliminations.
3. The document provides examples of amine syntheses such as reductive amination and