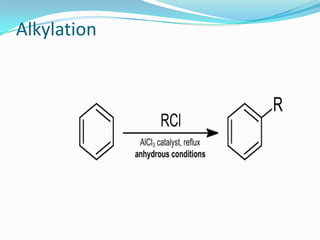
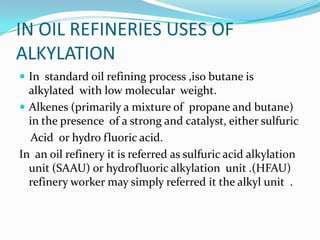
The document discusses alkylation, which is the transfer of an alkyl group from one molecule to another. It specifically discusses alkylation processes used in oil refineries, where iso-butane is alkylated with olefins. It describes nucleophilic, electrophilic, and carbene alkylating agents and their mechanisms. It also discusses sulfuric acid and hydrofluoric acid alkylation units used in refineries, and how cracking and polymerization can be combined with alkylation to increase gasoline yields from crude oil.