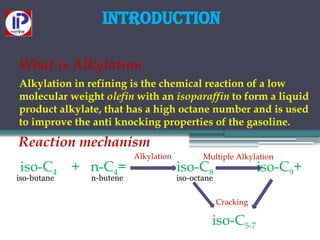
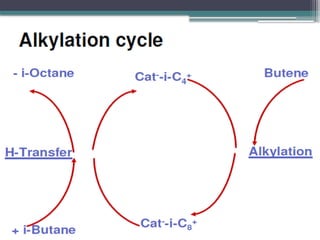
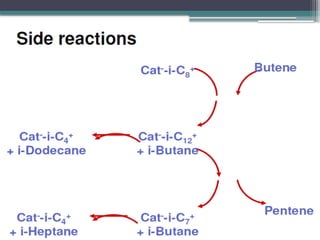
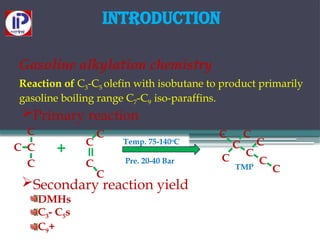
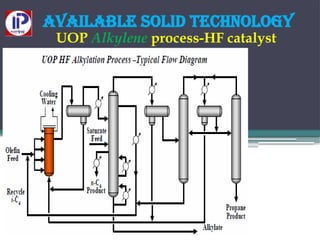
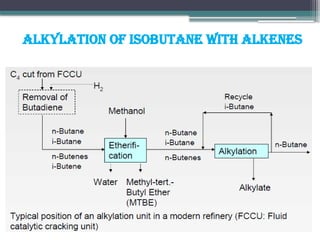
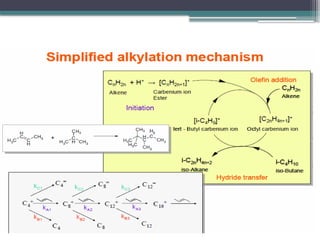
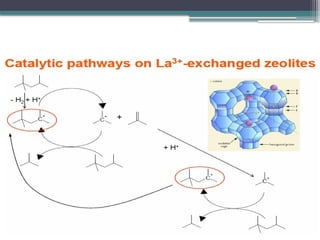
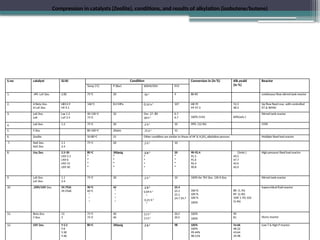
The document discusses the alkylation process in refining, focusing on the use of zeolite-based solid acid catalysts for alkylation of isobutane and butenes to produce high-octane gasoline. It covers various catalyst technologies, environmental benefits, and optimal reaction conditions, highlighting the efficiency and advantages of solid acid catalysis in this process. Additionally, it details available solid catalyst technologies, including different zeolites and their specific applications and performance metrics.