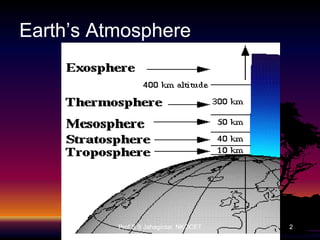
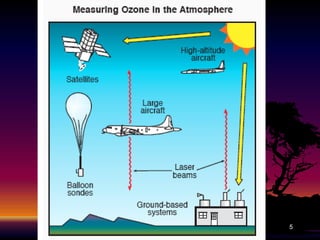
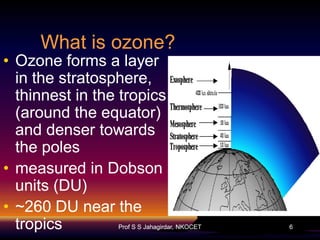
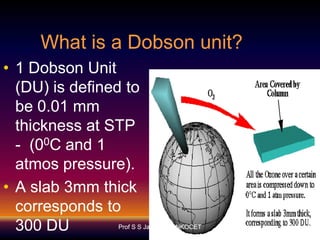
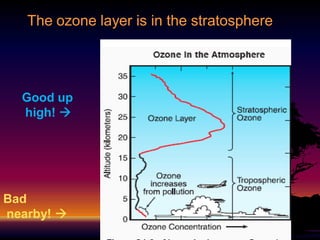
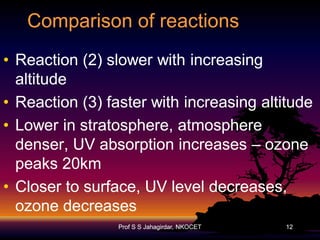
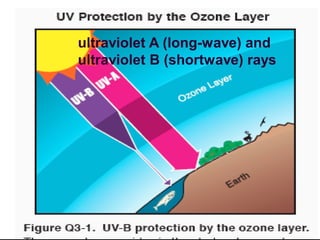
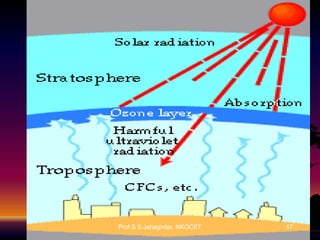
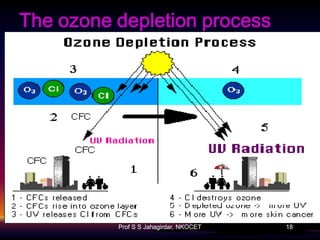
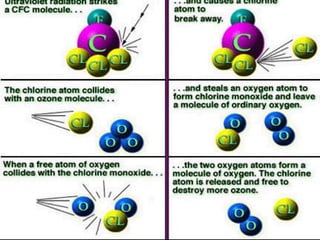
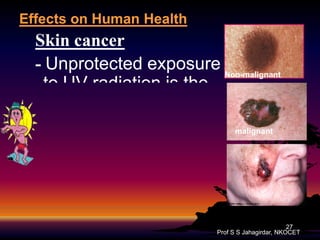
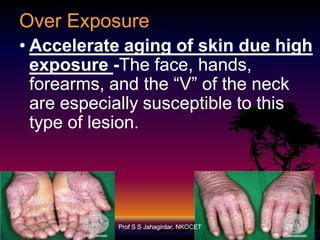
The document discusses the ozone layer, its formation, depletion caused by chlorofluorocarbons (CFCs), and their harmful effects on health and the environment. It highlights the protective role of ozone against ultraviolet radiation while detailing the adverse impacts of its depletion, including increased skin cancer, eye damage, and threats to ecosystems. Efforts such as the Montreal Protocol, aimed at reducing CFC usage, are mentioned as steps towards addressing ozone depletion, though challenges remain due to the long-lasting effects of chemicals already emitted.