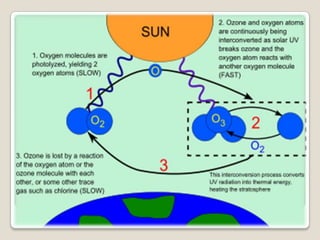
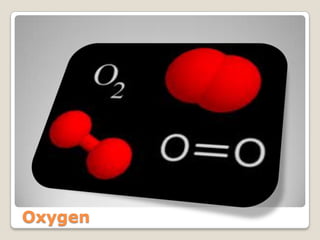
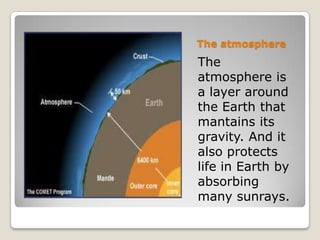
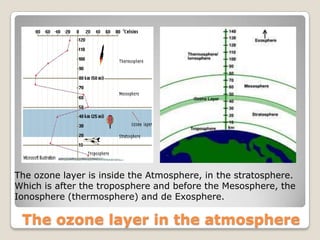
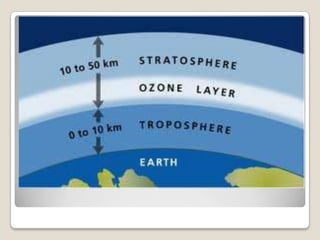
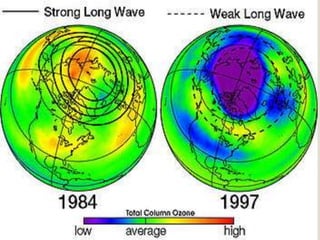
The ozone layer is a layer in the Earth's atmosphere that contains relatively high concentrations of ozone. It was discovered in 1913 and protects life on Earth by absorbing harmful ultraviolet radiation from the sun. Depletion of the ozone layer is caused by chemicals like chlorofluorocarbons that were used in aerosol sprays and refrigerants until their ban in the late 1970s. Ozone depletion increases UV radiation at the surface of the Earth and can potentially damage skin, eyes, and suppress immune systems.