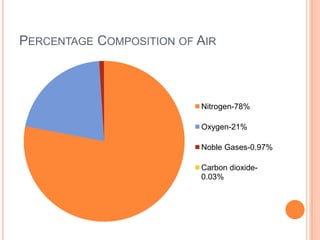
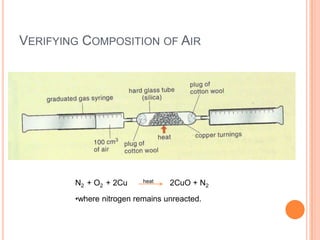
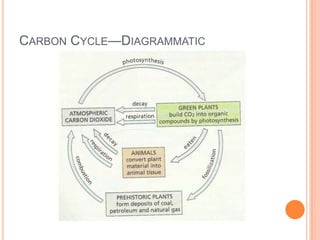
1) The document discusses the composition of air and various methods to analyze its components, such as the copper oxide method to verify nitrogen content.
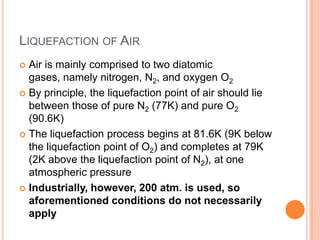
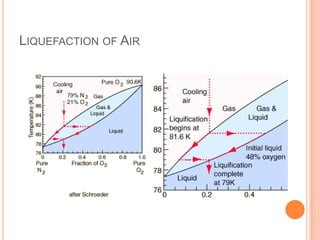
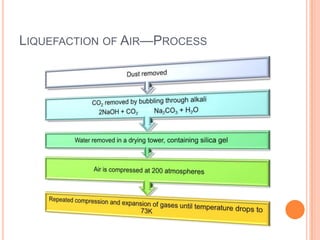
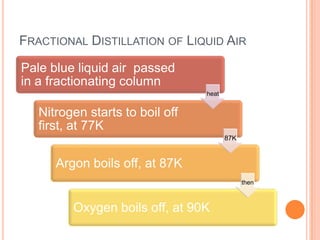
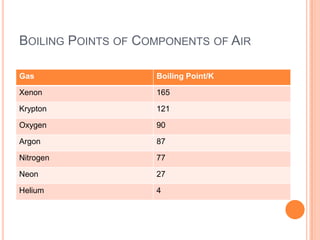
2) It also covers topics like the liquefaction process of air using fractional distillation to separate components by boiling point.
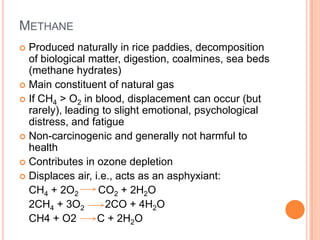
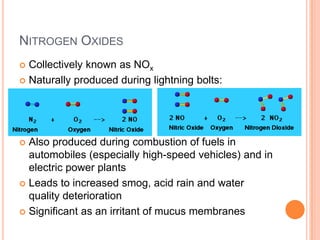
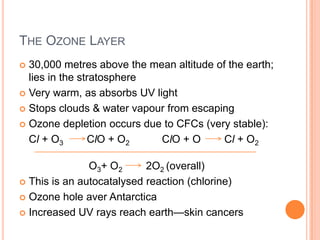
3) Additionally, it summarizes several primary air pollutants like carbon monoxide, methane, nitrogen oxides, ozone, and sulfur dioxide; their sources and health effects; and some remedies for air pollution control.