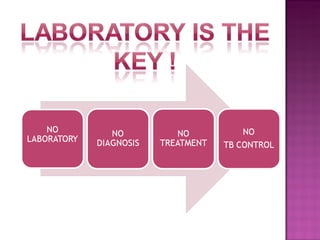
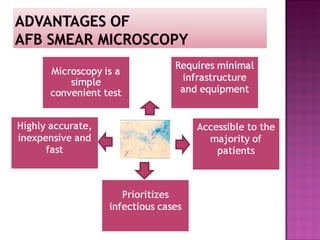
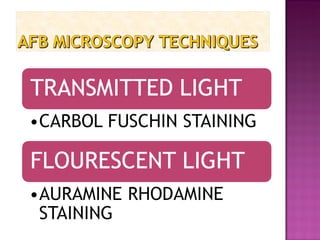
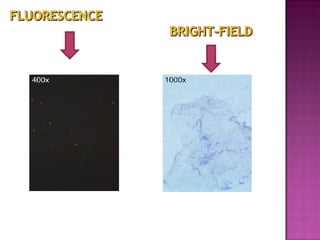
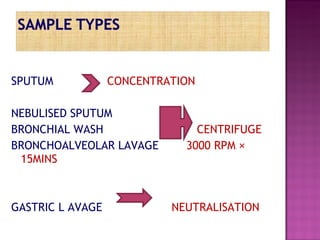
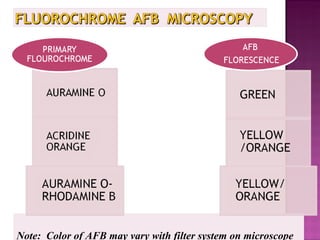
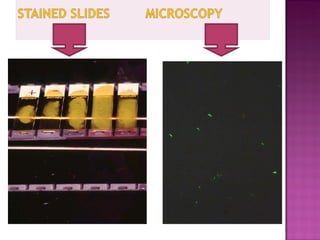
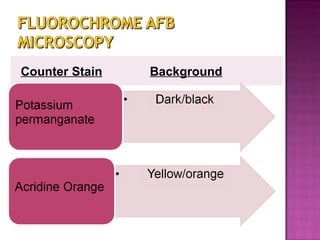
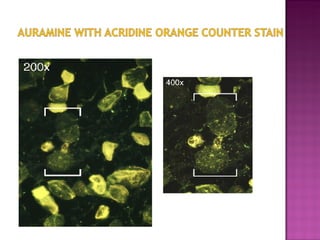
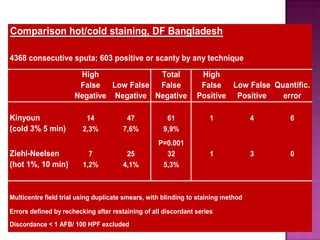
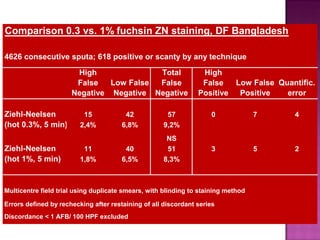
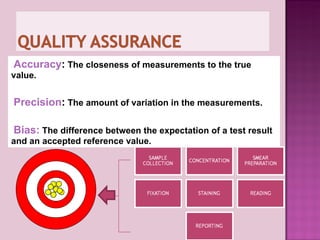
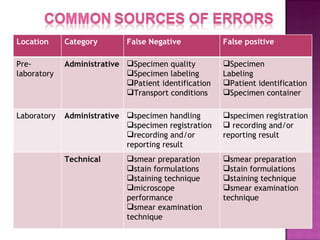
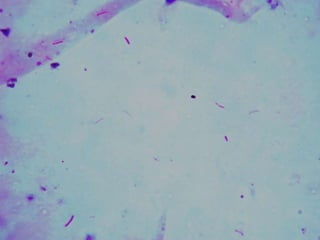
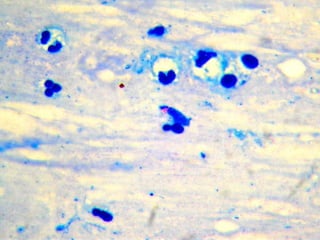
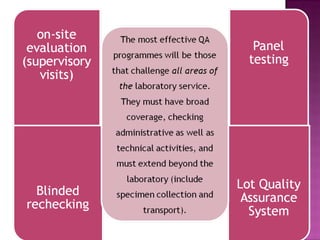
The document provides instructions for sputum smear microscopy for acid-fast bacilli (AFB) detection. It describes sample collection and processing including centrifugation. Methods for smear preparation, staining using carbol fuschin and decolorization are outlined. The importance of laboratory safety, quality assurance, and the role of microscopy in diagnosis are briefly discussed.