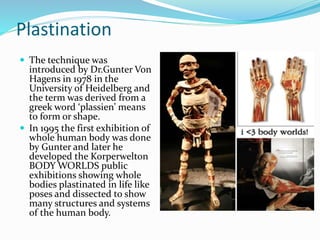
This document discusses the technique of plastination as an alternative to formalin for preserving gross specimens used in teaching oral pathology. It describes the limitations of formalin preservation and provides details on the plastination process, which involves dehydrating tissue, removing lipids, and infiltrating the tissue with curable polymers like silicone. The technique produces specimens that are odorless, non-toxic, and durable for long-term storage and examination. Plastinated specimens are well-suited for teaching as they are easy to handle and interpret structural features. While more complex than formalin preservation, plastination produces high-quality specimens without many of the drawbacks of traditional methods.