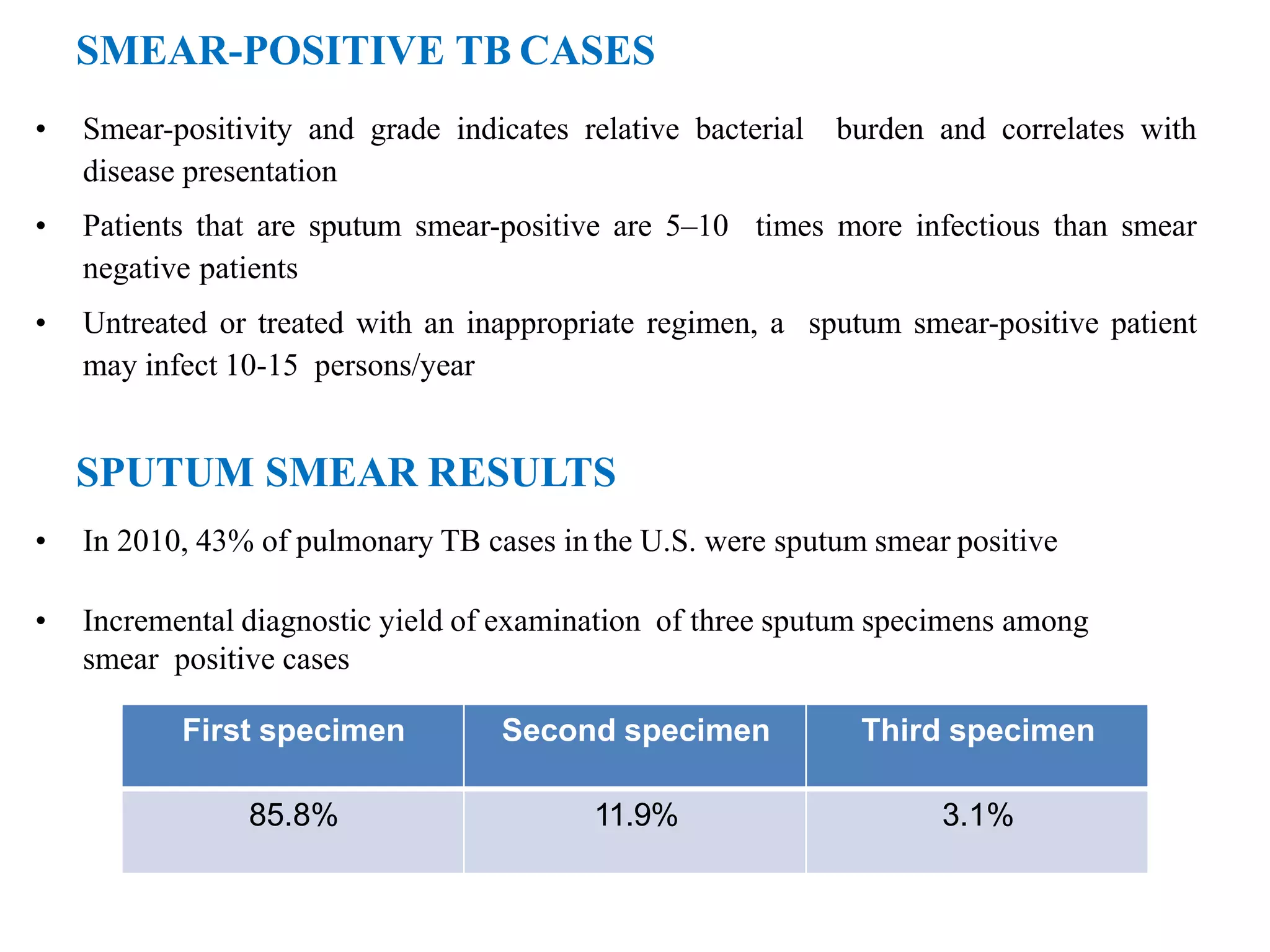
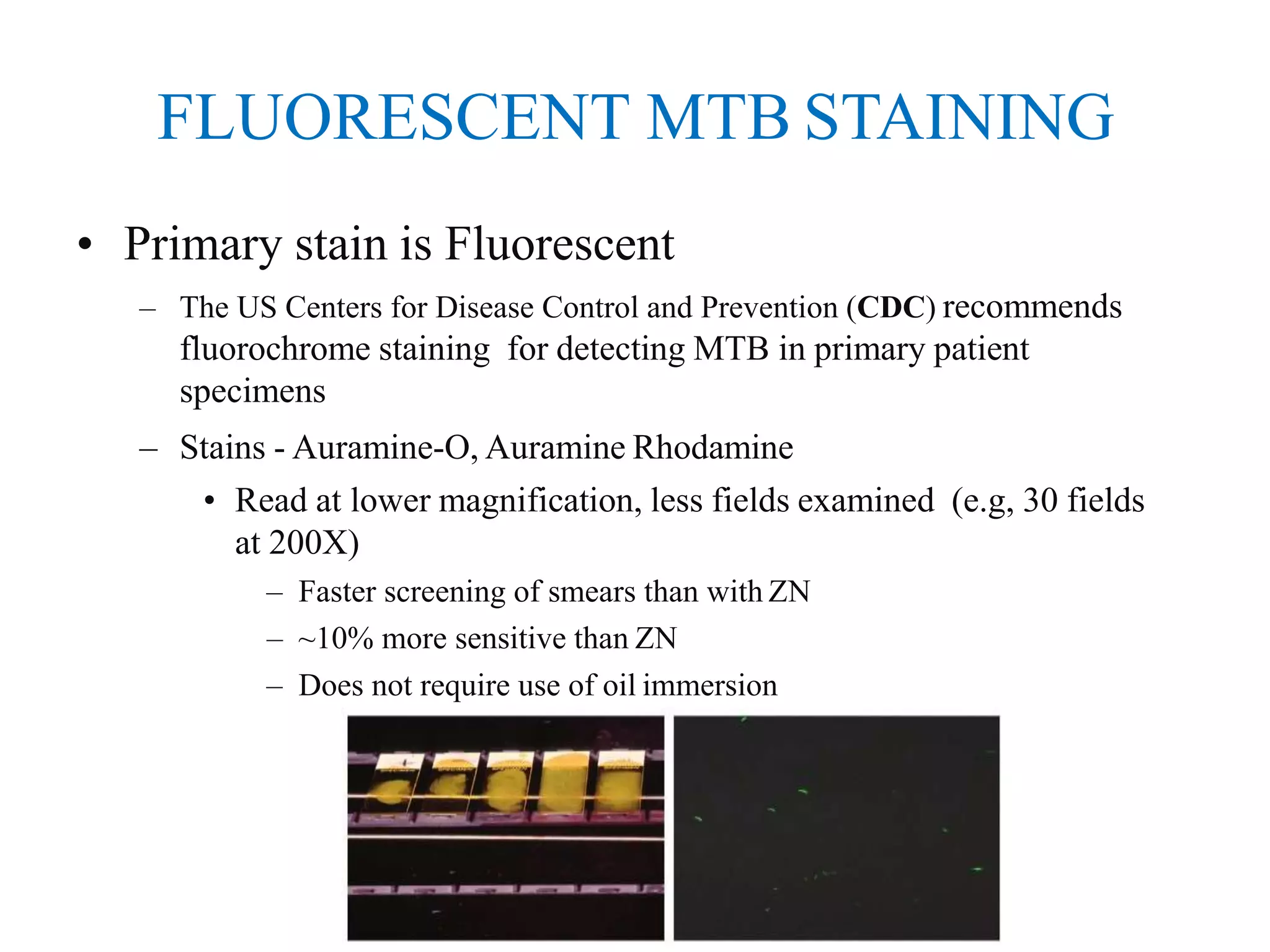
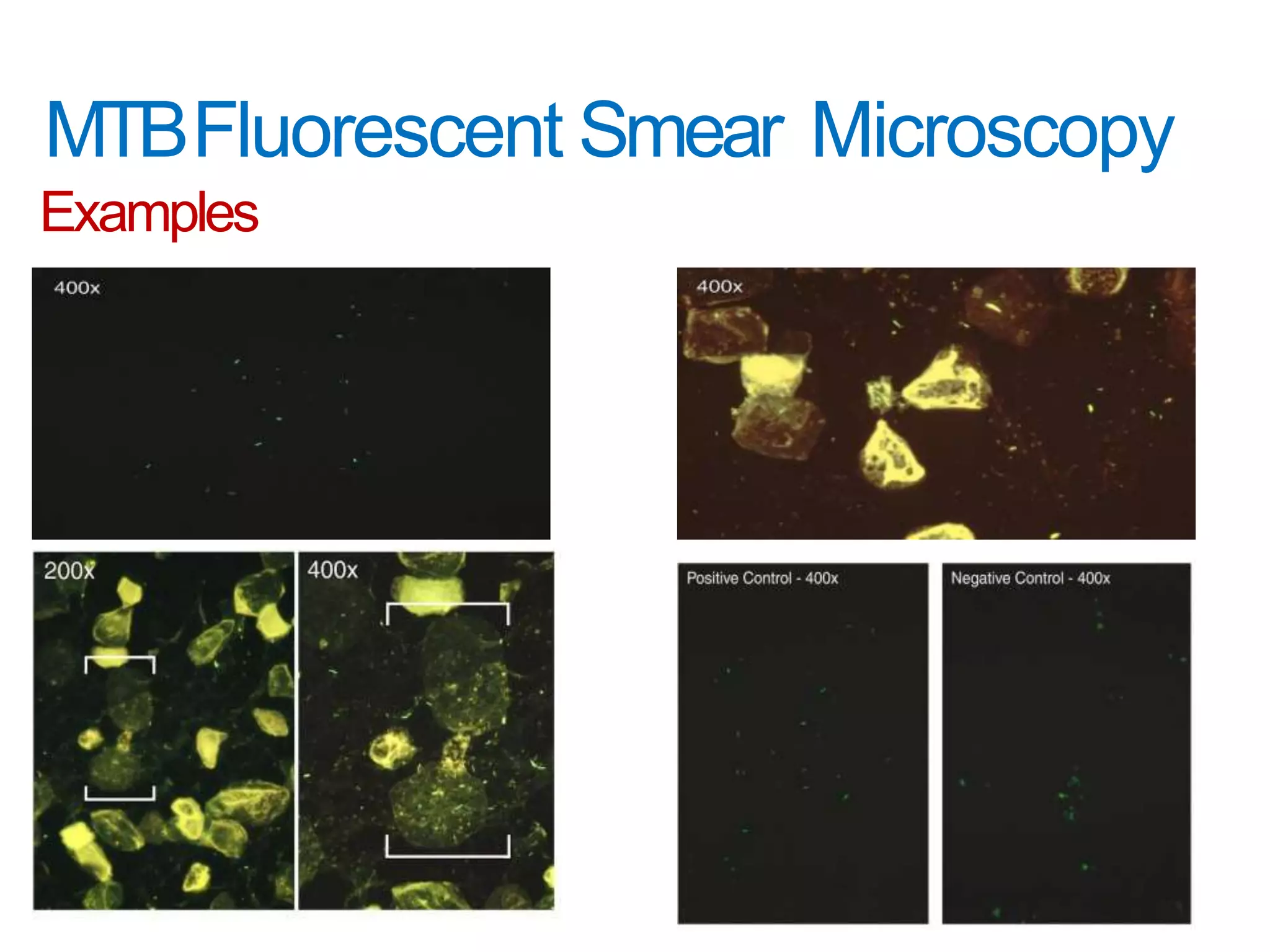
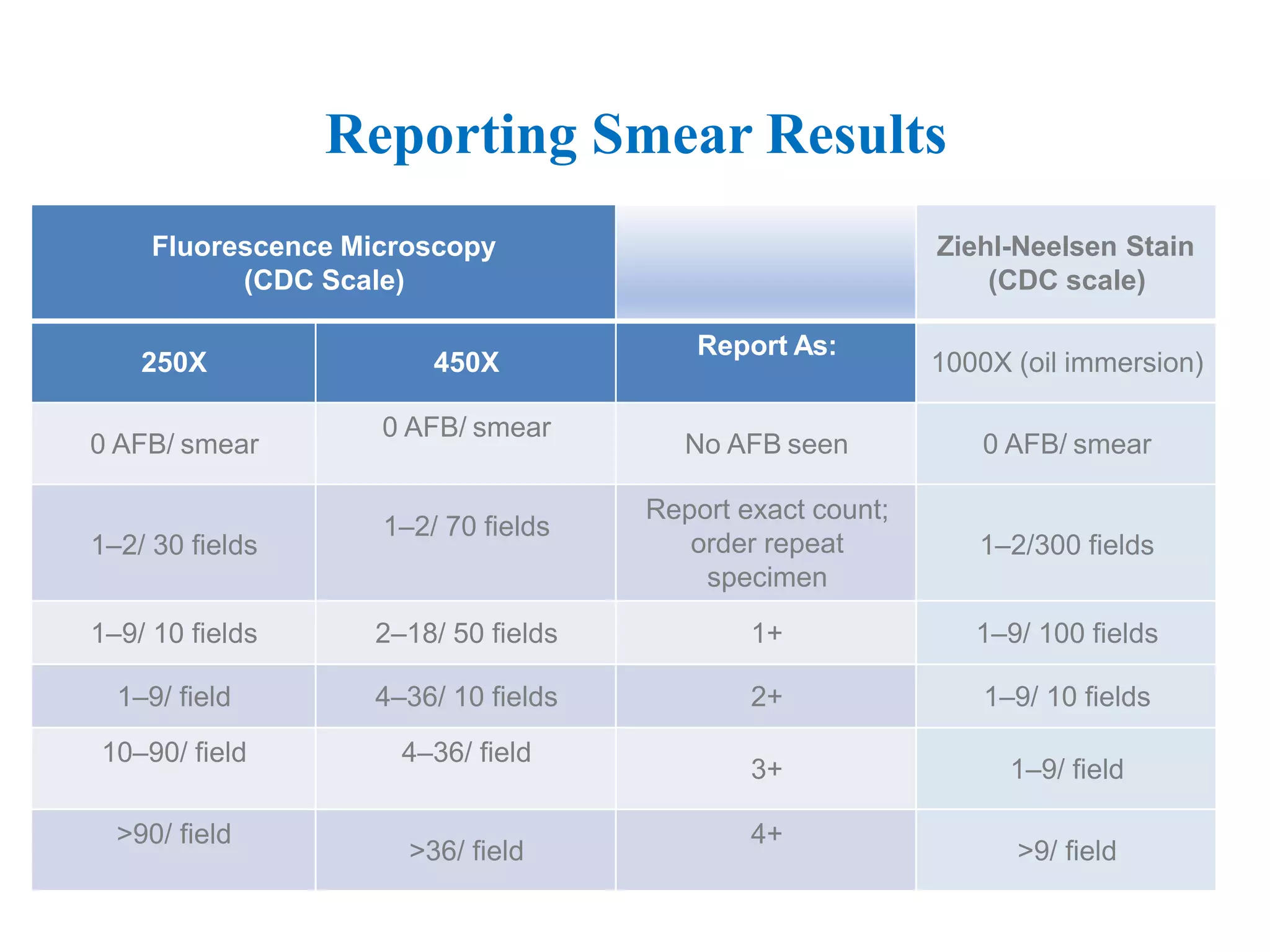
This document provides information on the microscopic method for detecting Mycobacterium tuberculosis (MTB) in smears. It discusses how acid-fast bacilli, like MTB, retain staining due to their lipid-rich cell walls. The document outlines the advantages and disadvantages of smear microscopy, and describes Ziehl-Neelsen and fluorescent staining techniques. It emphasizes the importance of systematic examination of stained smears under the proper magnification and a minimum number of fields to accurately detect MTB.