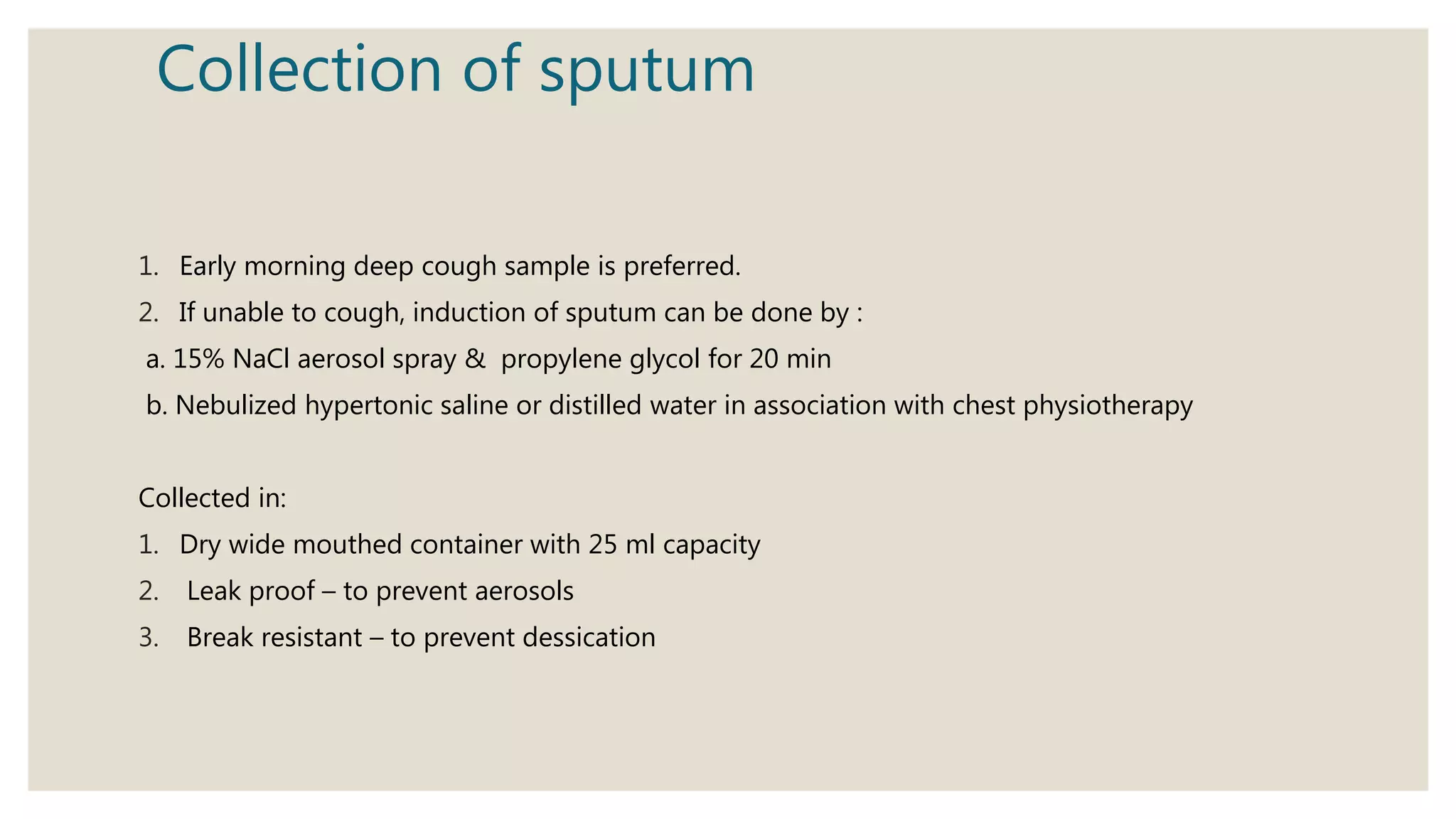
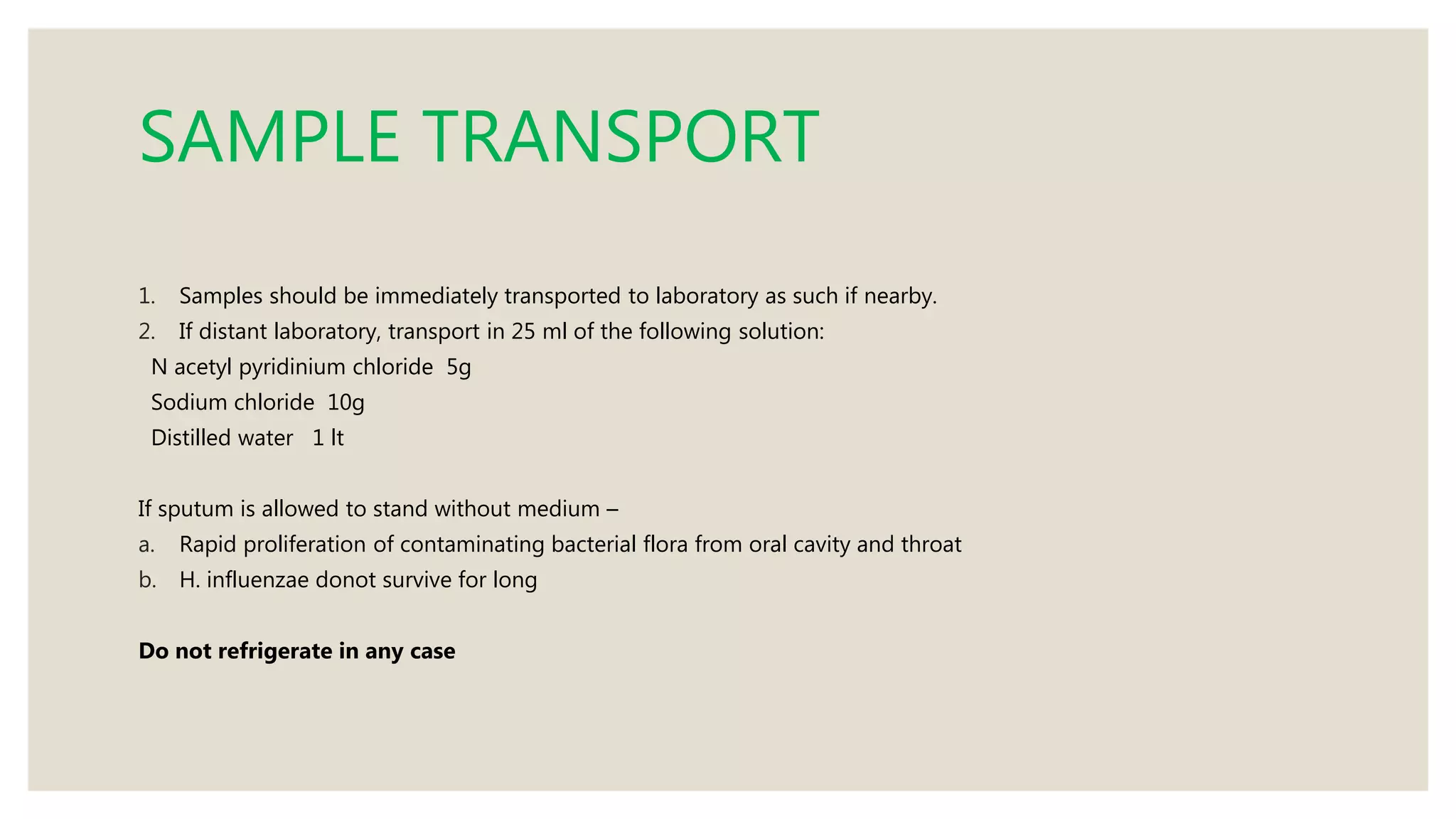
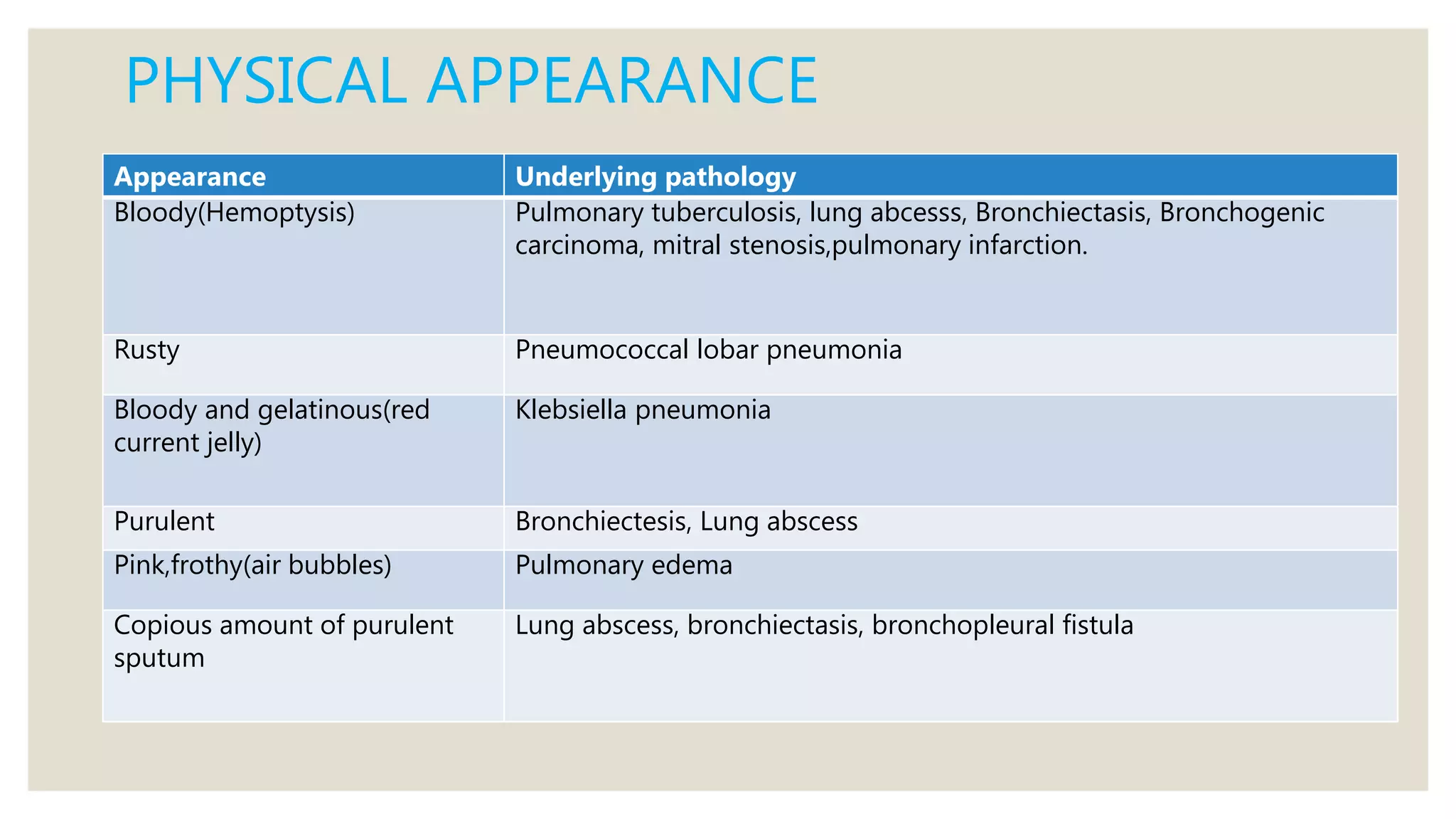
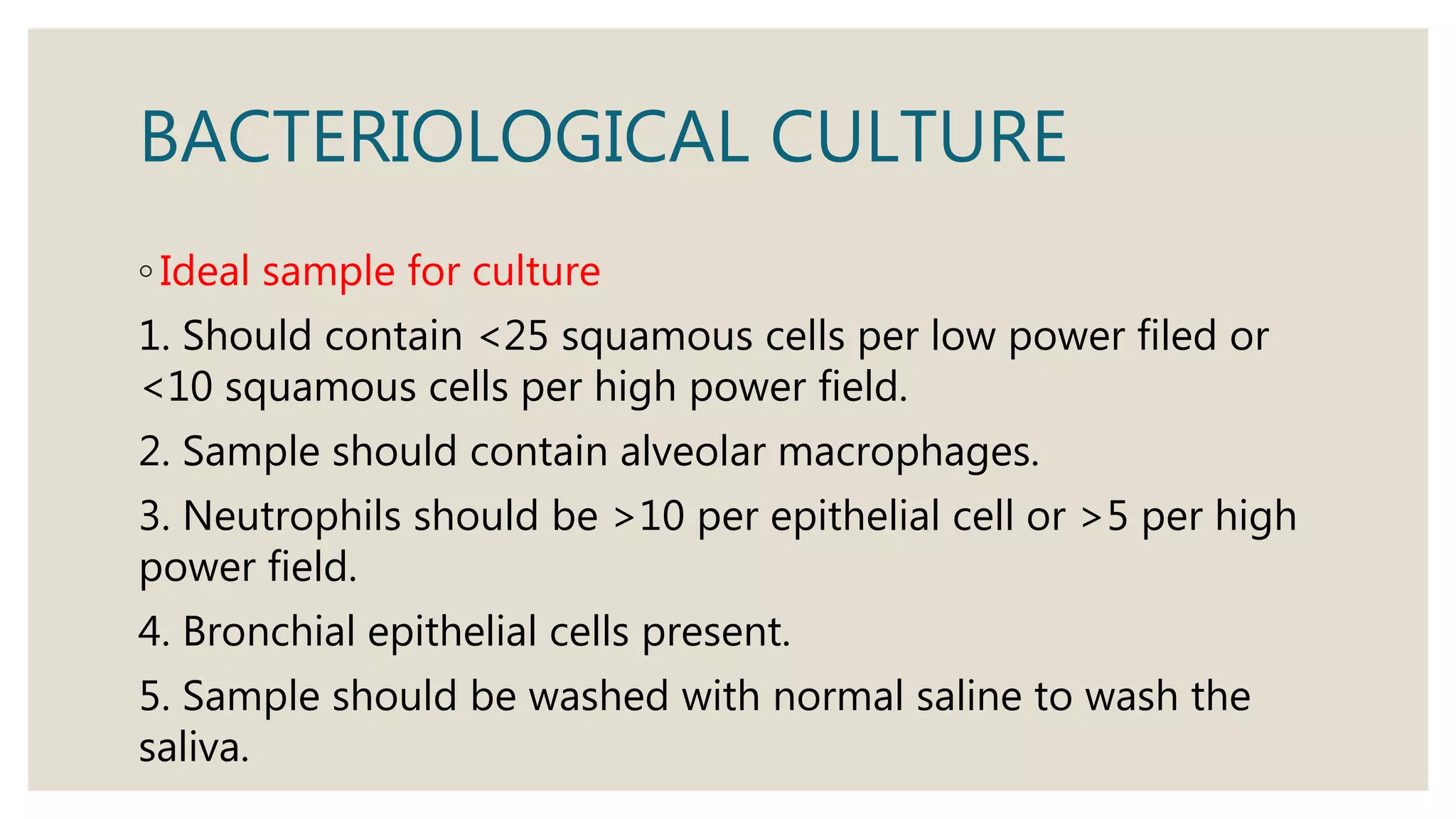
Sputum examination provides important diagnostic information by analyzing material coughed up from the lungs and respiratory tract. Key indications for sputum examination include identifying the causative organism in suspected lower respiratory infections like pneumonia or tuberculosis. Sputum samples can also be examined cytologically to detect malignant cells or investigate other infections. Proper collection and transport of sputum samples is important for microbiological culture and other tests. Staining and microscopic examination of sputum looks for bacteria, fungi, parasites and other pathogenic organisms. Molecular tests like PCR provide a rapid and sensitive method for tuberculosis diagnosis.