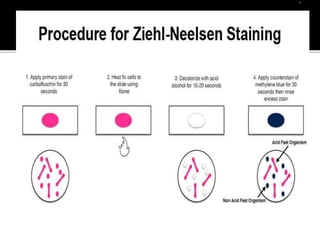
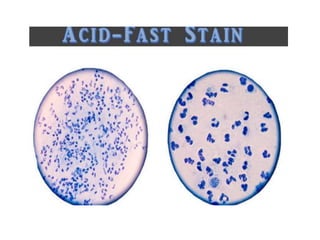
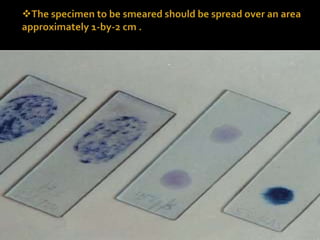
The document provides detailed instructions for performing the Ziehl-Neelsen acid-fast staining technique used to identify Mycobacteria. It describes preparing staining solutions such as carbol fuchsin and acid alcohol. The staining procedure involves heat fixing a smear, staining it with carbol fuchsin, decolorizing with acid alcohol, counterstaining and examining under a microscope. Mycobacteria appear red against a blue background. The document also notes some challenges with the technique like avoiding false positive or negative results.