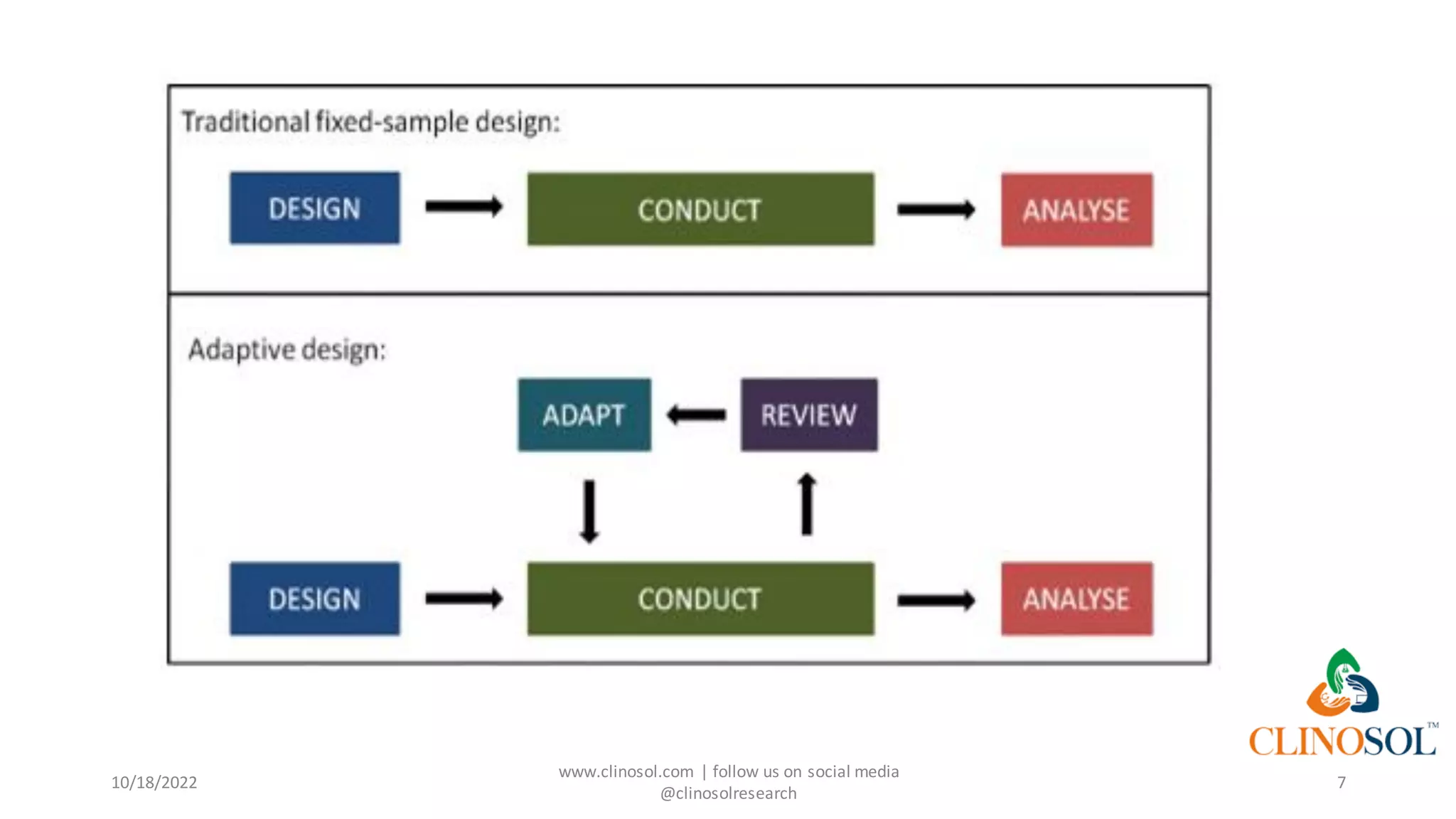
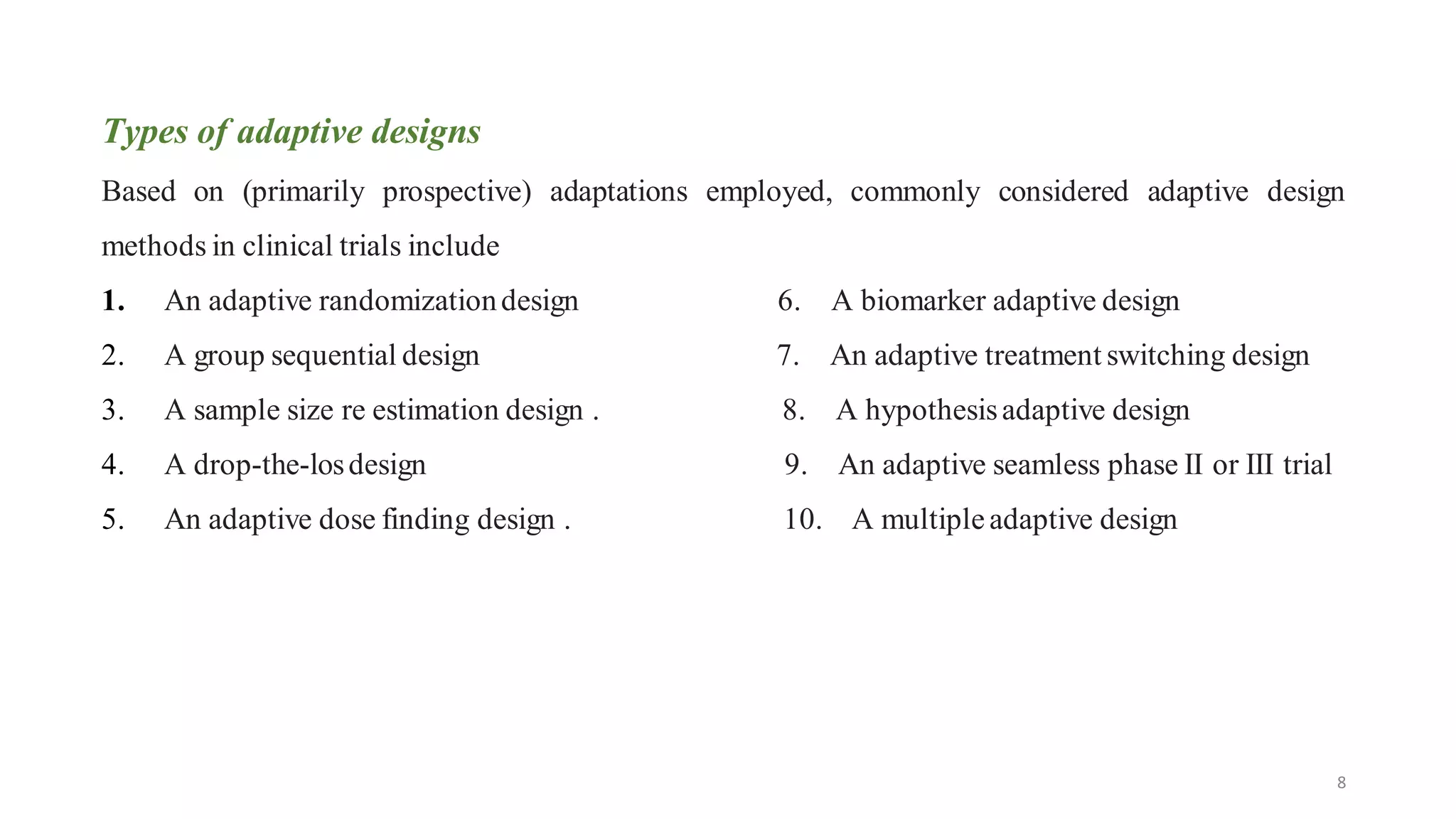
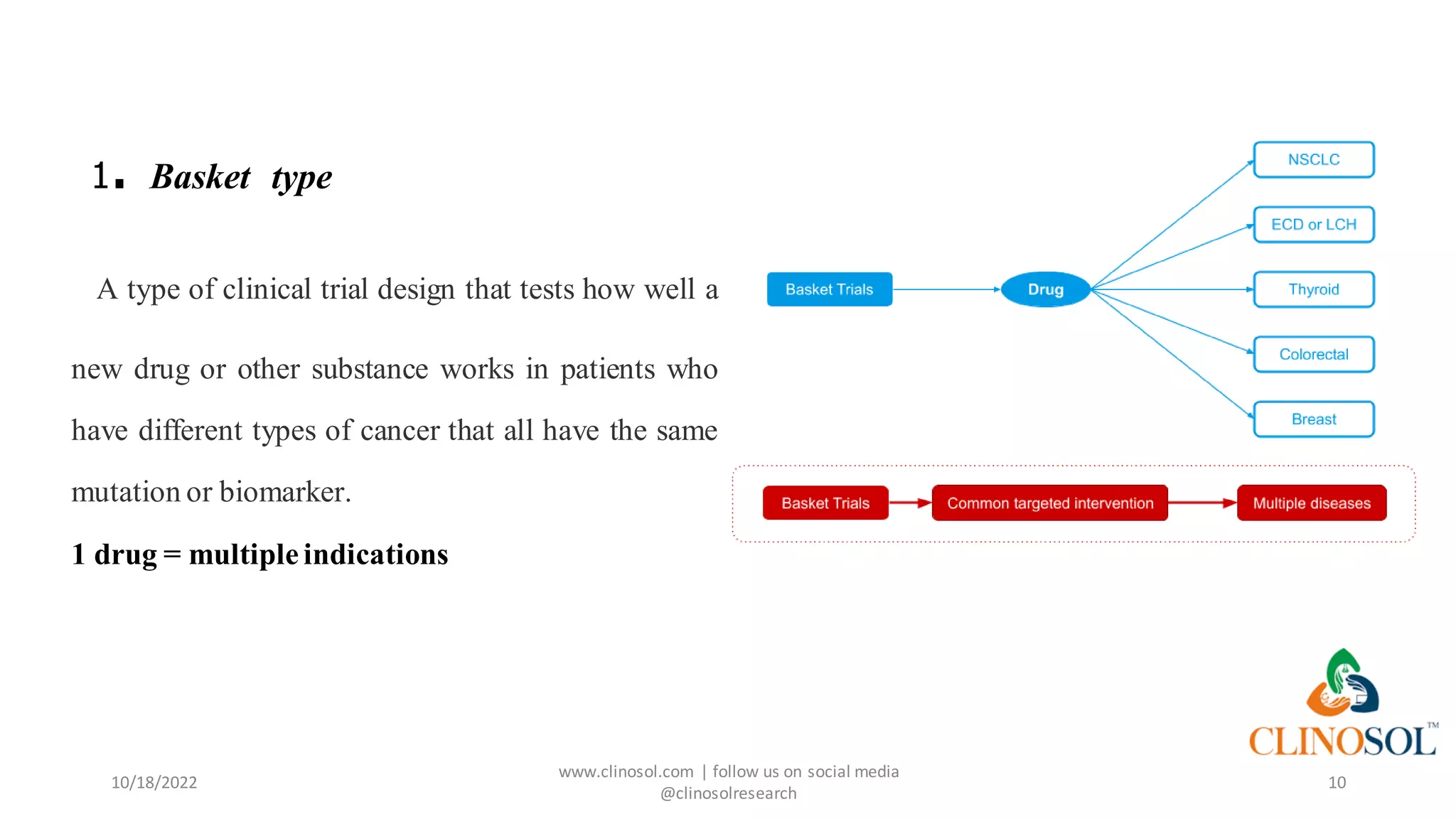
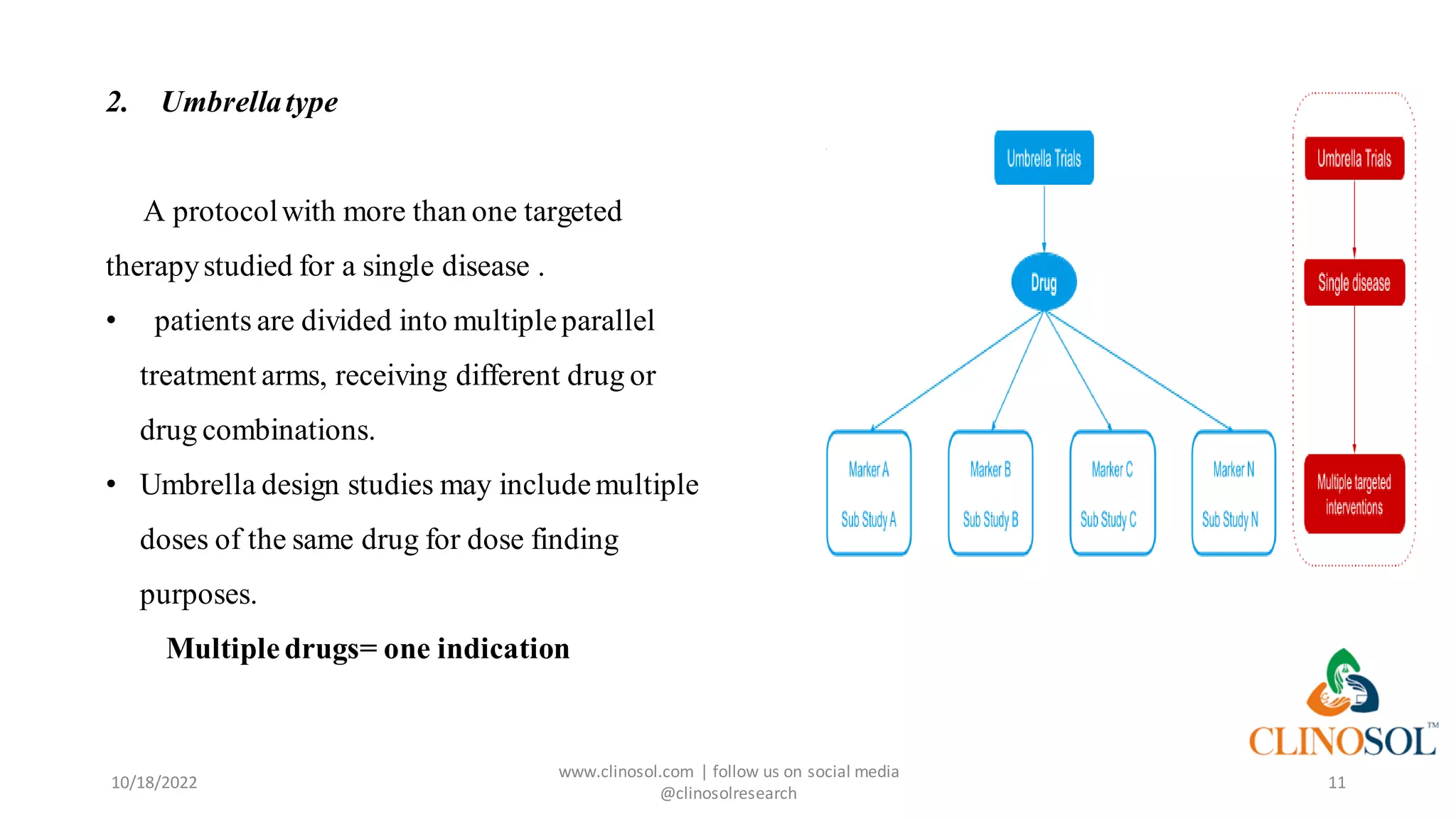
The document presents advanced clinical trial designs, specifically focusing on adaptive designs and master protocols, which enhance the efficiency and effectiveness of drug development. It discusses the advantages and disadvantages of these designs, emphasizing their potential to optimize research processes while managing costs and biases. Ultimately, it highlights the transformation of drug development through innovative trial methodologies, requiring collaborative alignment among stakeholders.