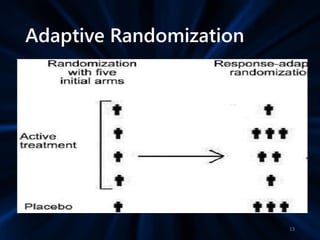
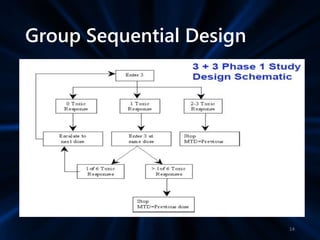
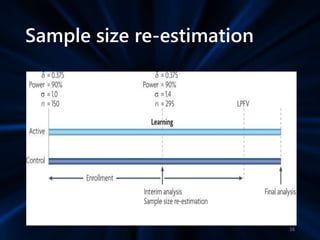
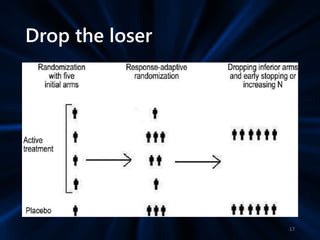
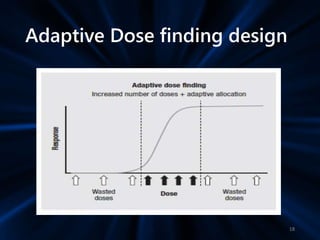
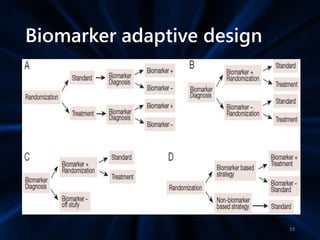
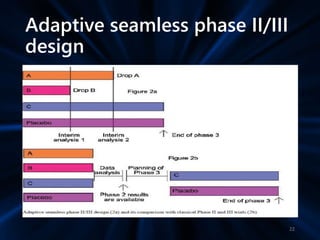
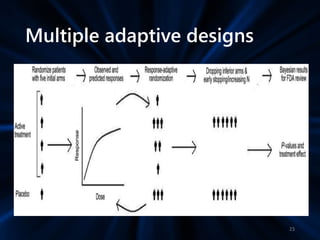
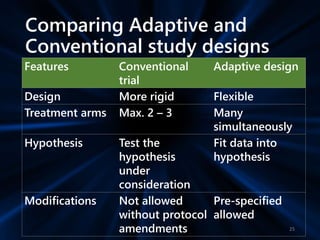
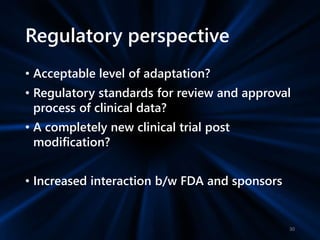
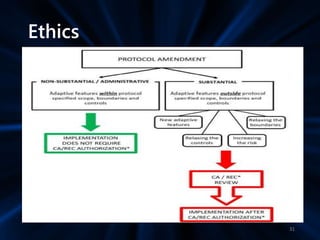
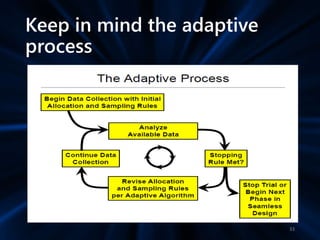
Adaptive study designs allow for prospectively planned modifications to the design based on interim data analysis in order to increase efficiency. This is more flexible than conventional designs but also more complex. Key types of adaptations include sample size re-estimation, dropping treatment arms, and adapting doses or endpoints. Advantages include obtaining the same information more efficiently and improving understanding of treatment effects. However, concerns relate to increased type I error rates and challenges in interpretation. Regulatory perspectives are still evolving around adaptive designs. Careful planning and control mechanisms are needed to balance flexibility with scientific integrity.