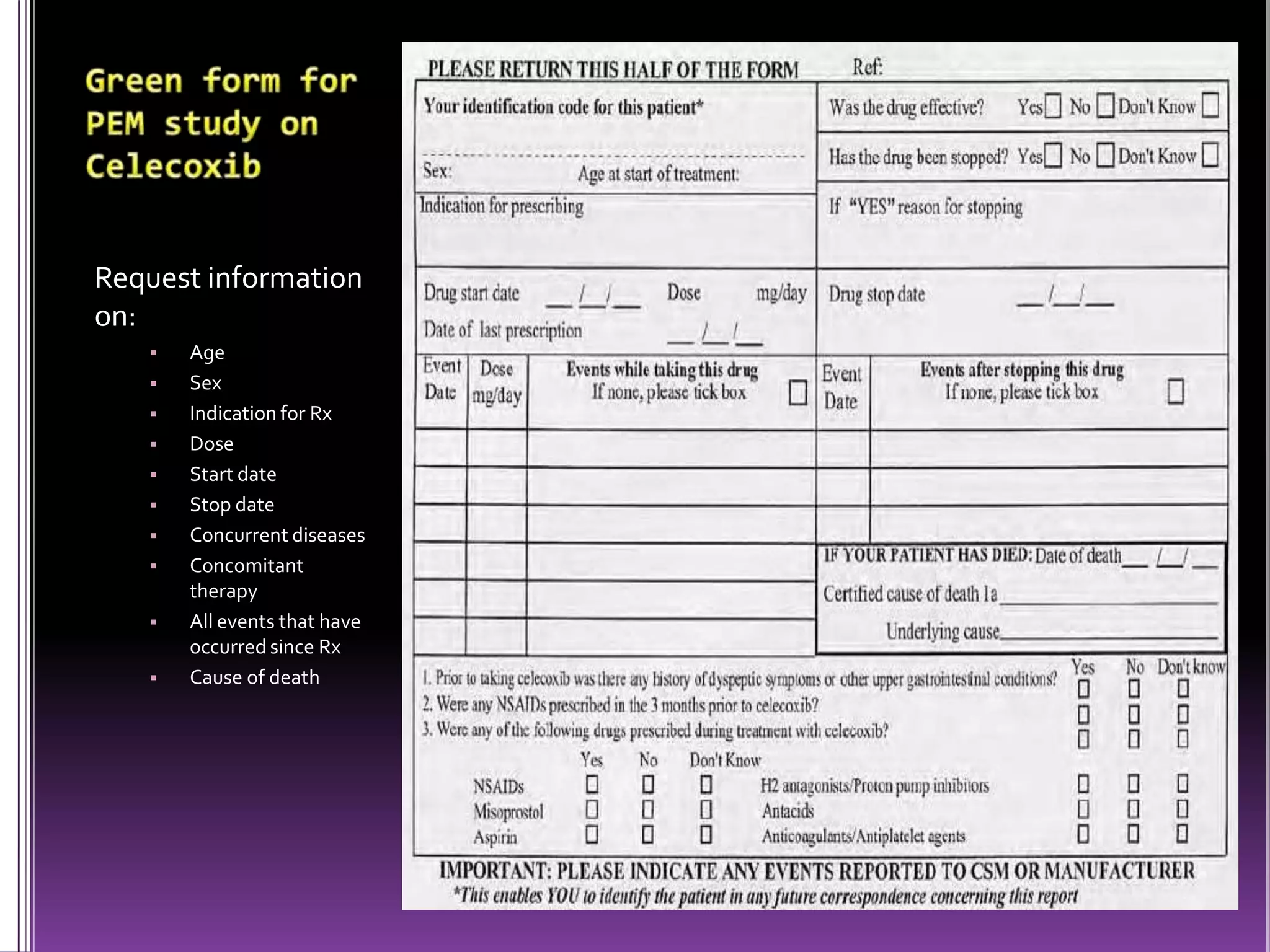
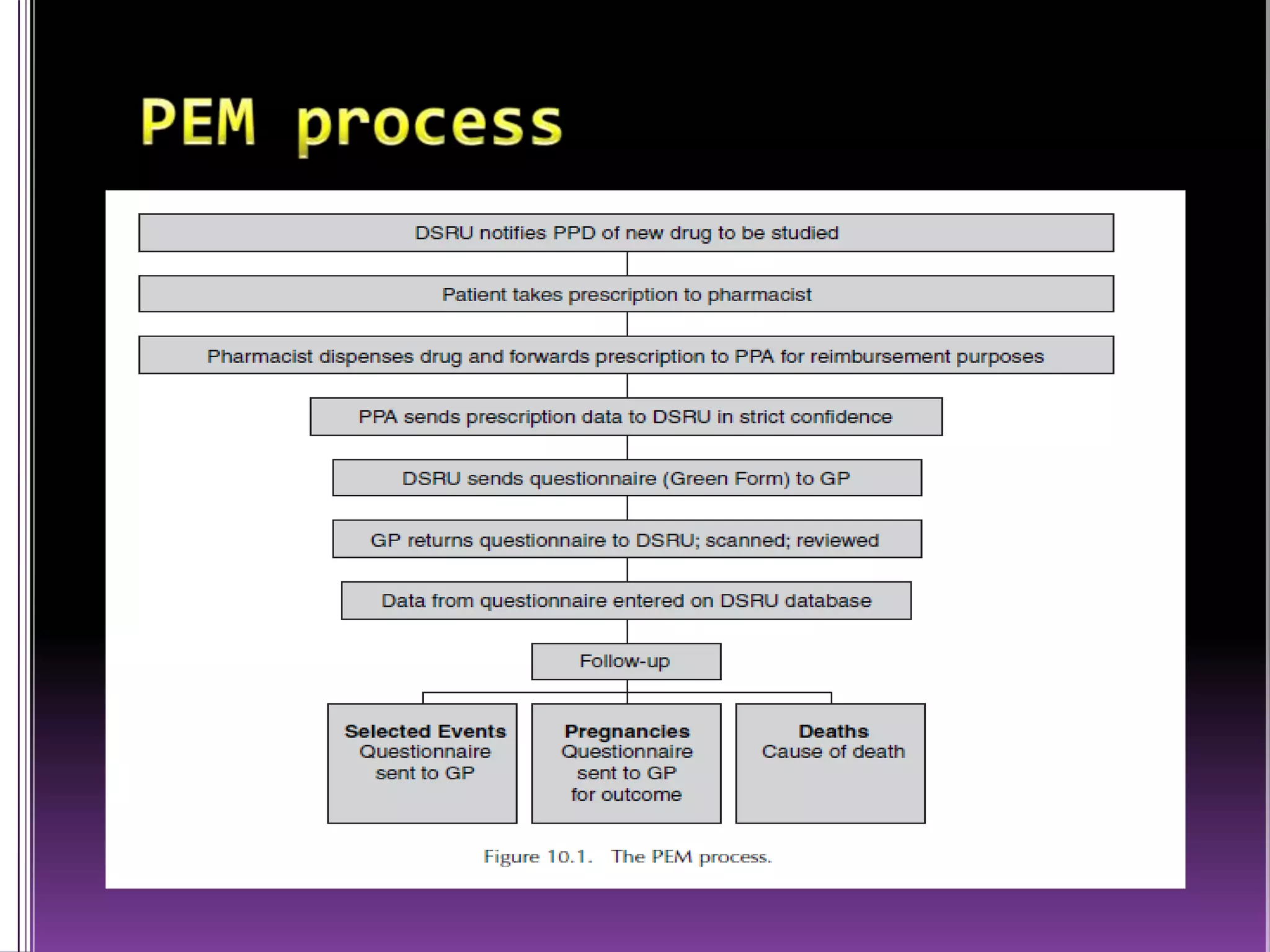
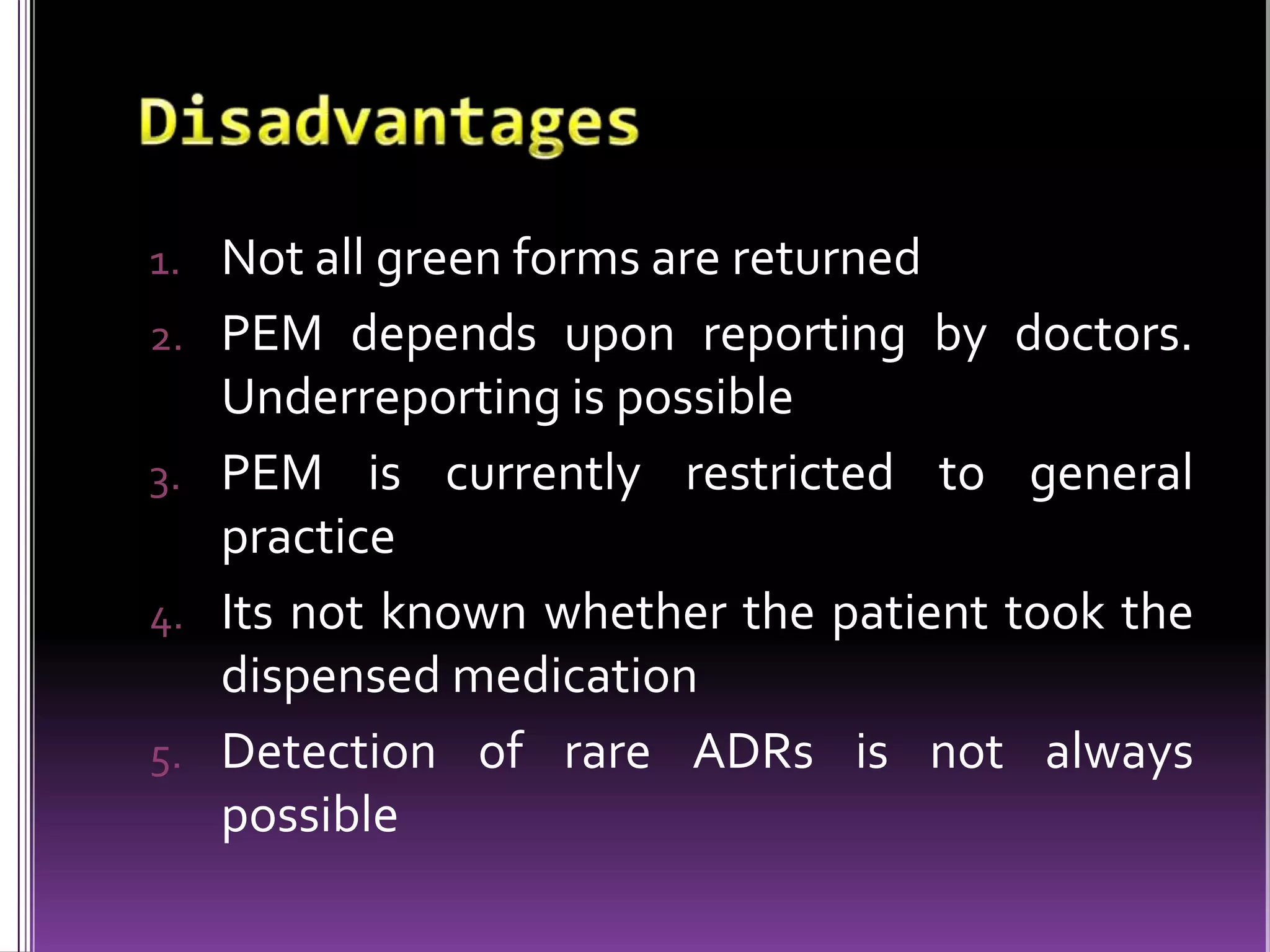
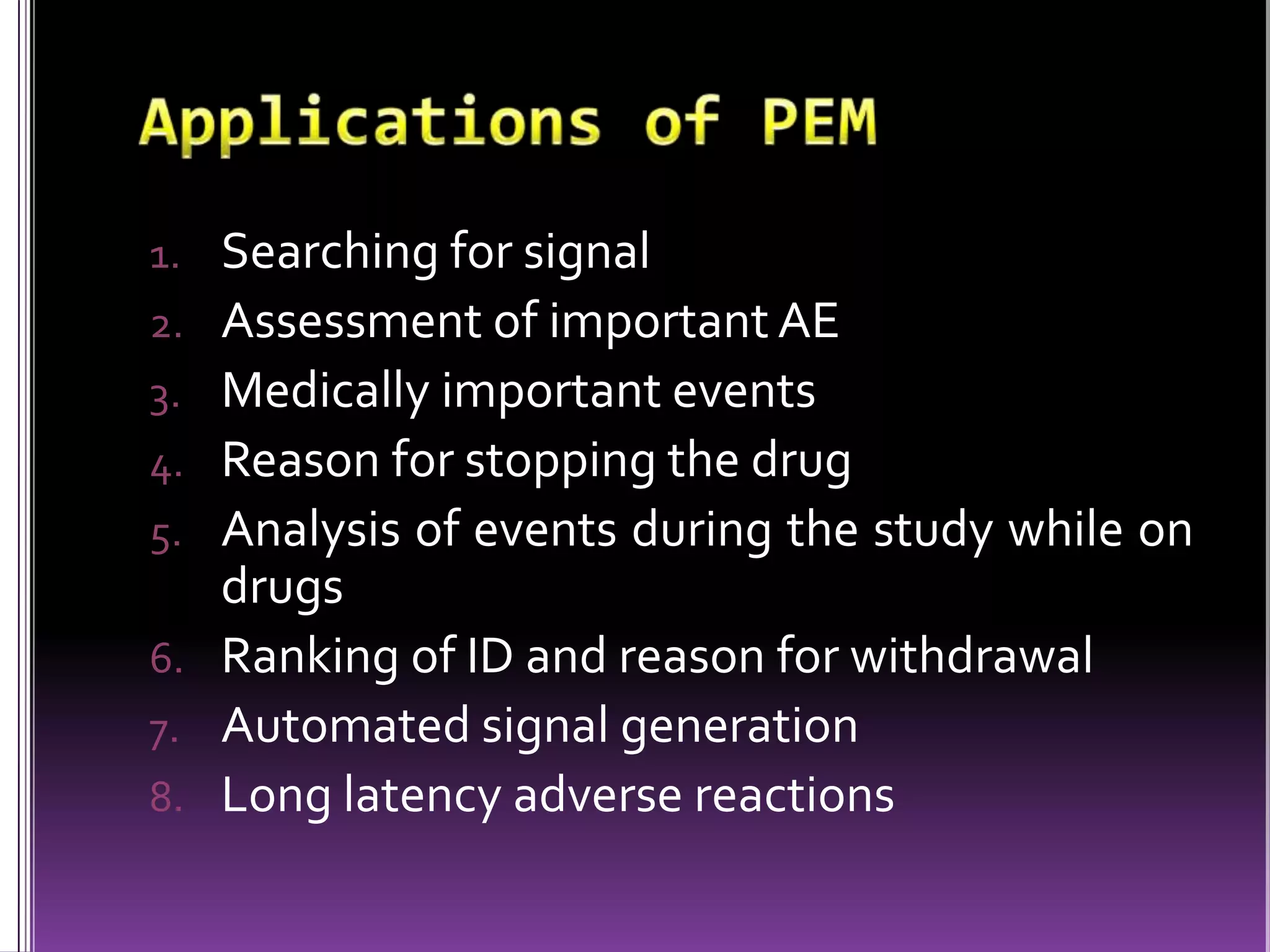
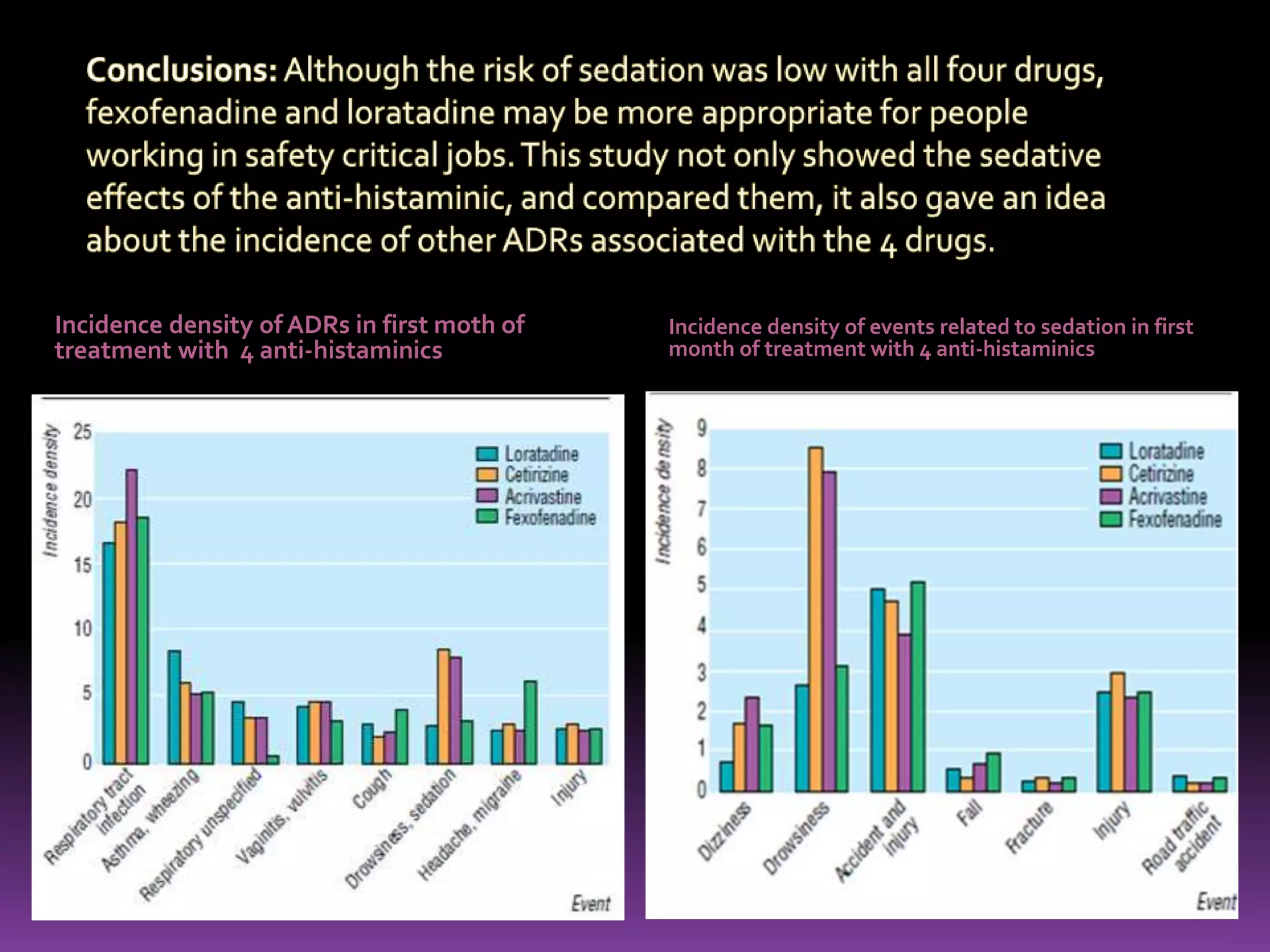
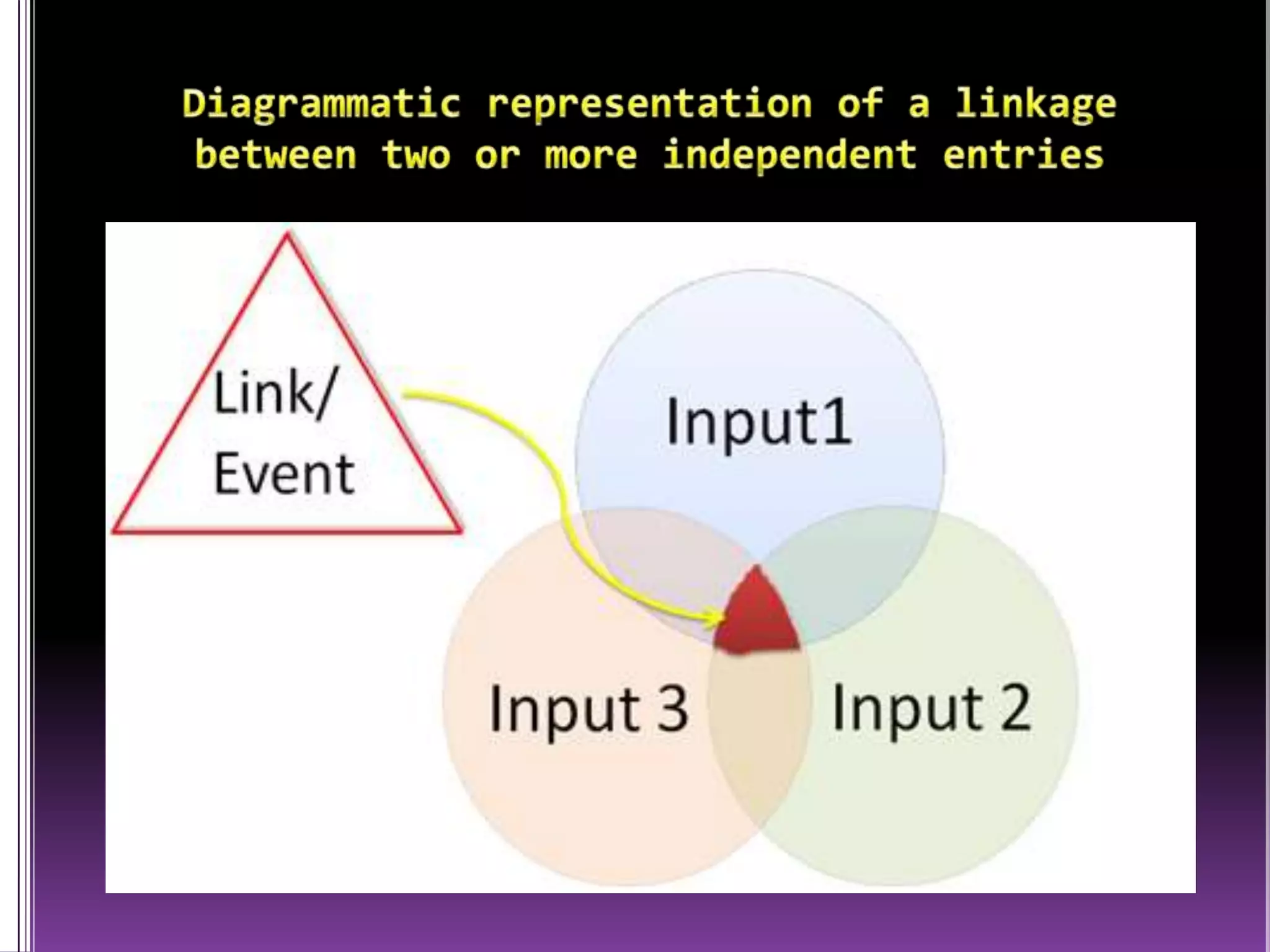
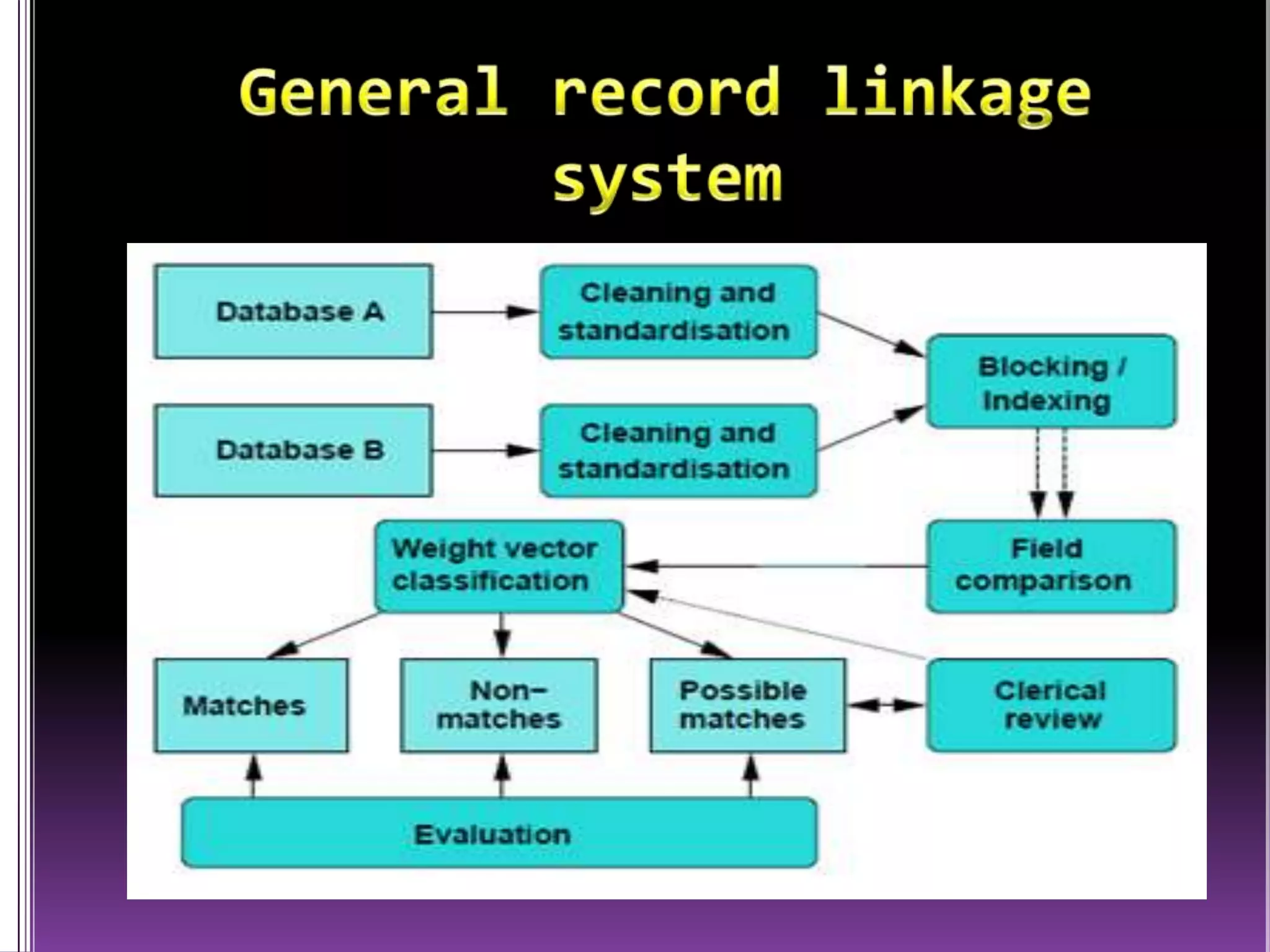
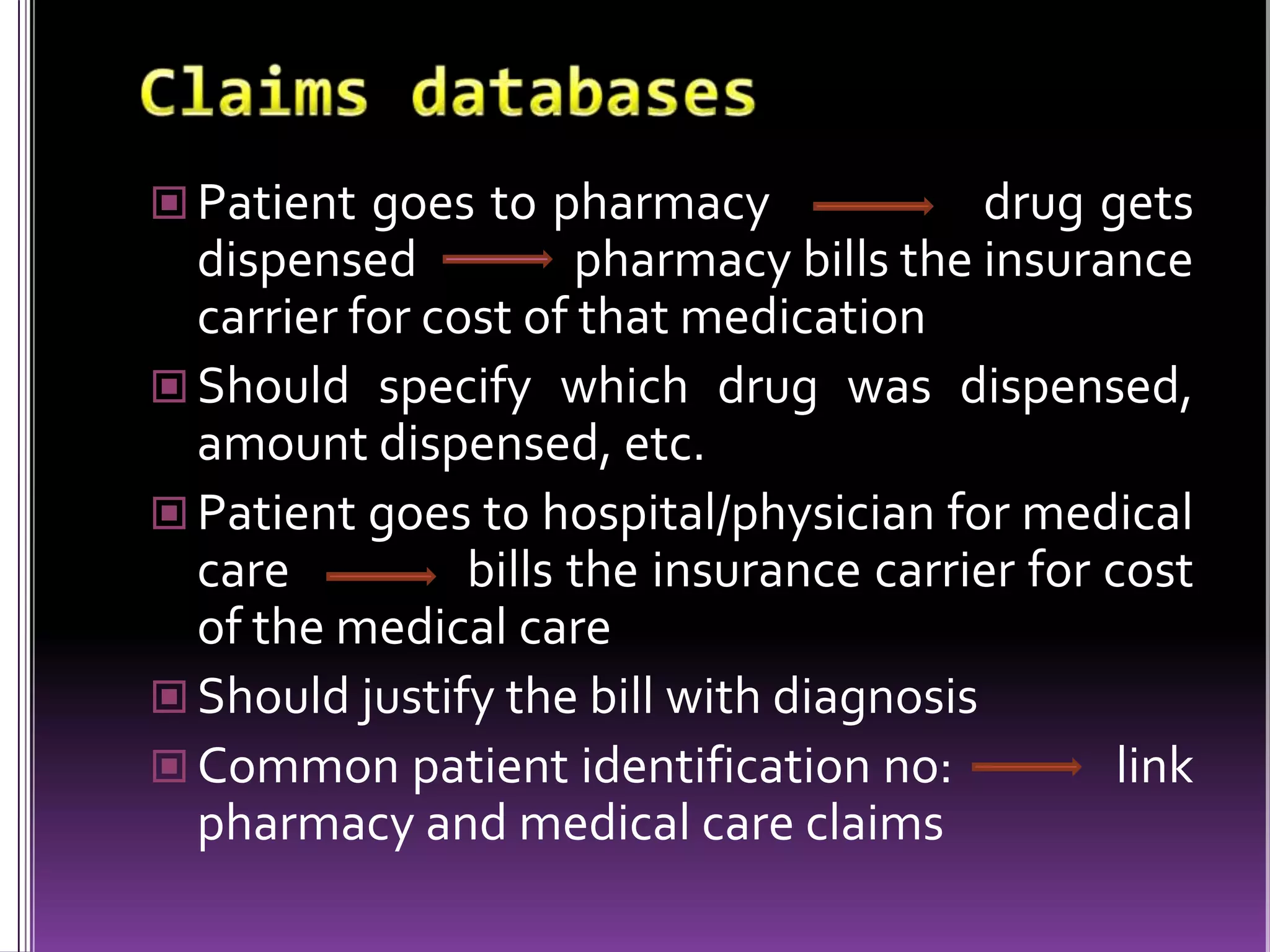
This document discusses record linkage and its use in pharmacovigilance. Record linkage involves combining records from different data sources that relate to the same individual to create a single longitudinal record. It allows rapid access to a patient's complete medical history across different data sources. This reduces the time needed to study relationships between drug exposure and health outcomes. Challenges include ensuring data quality and completeness when integrating records from various sources.