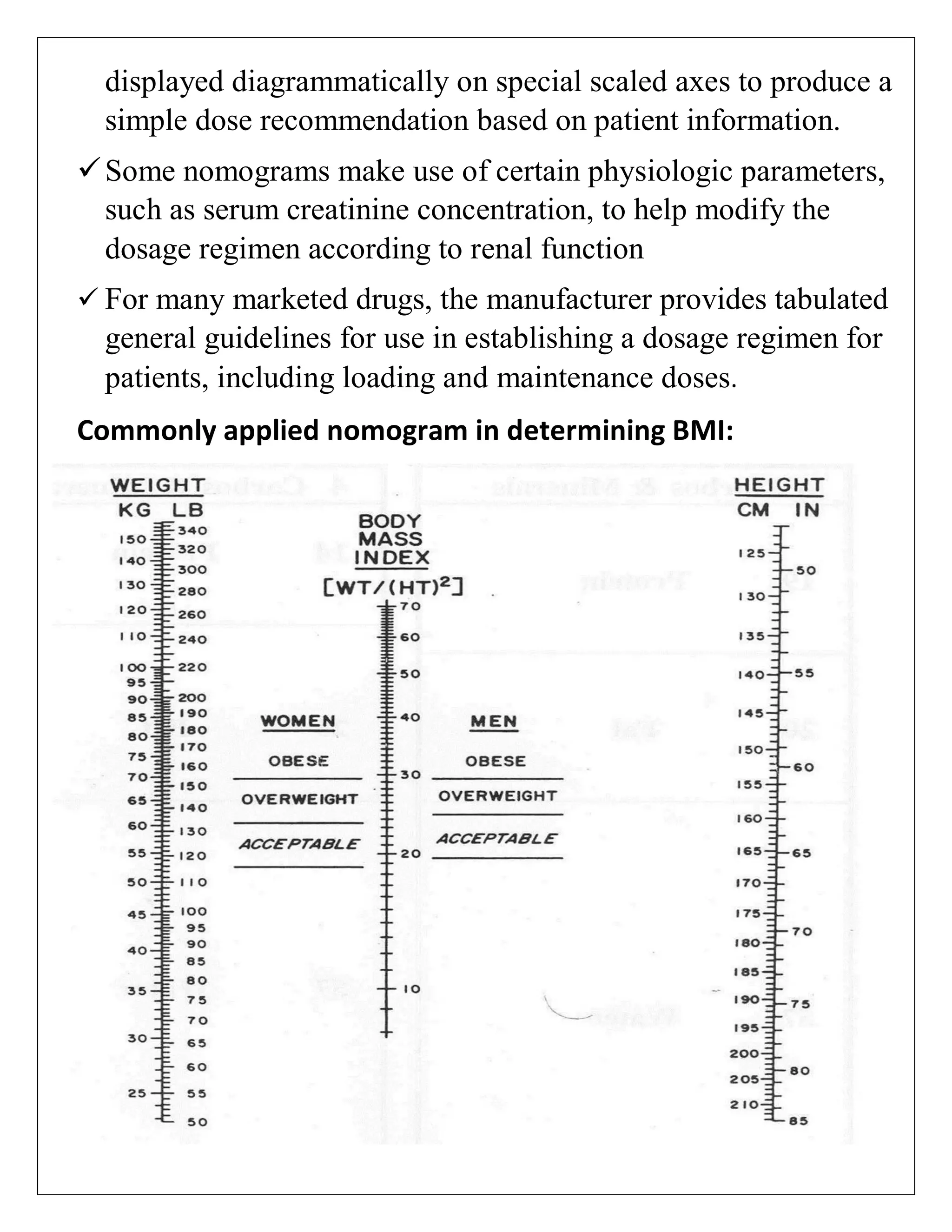
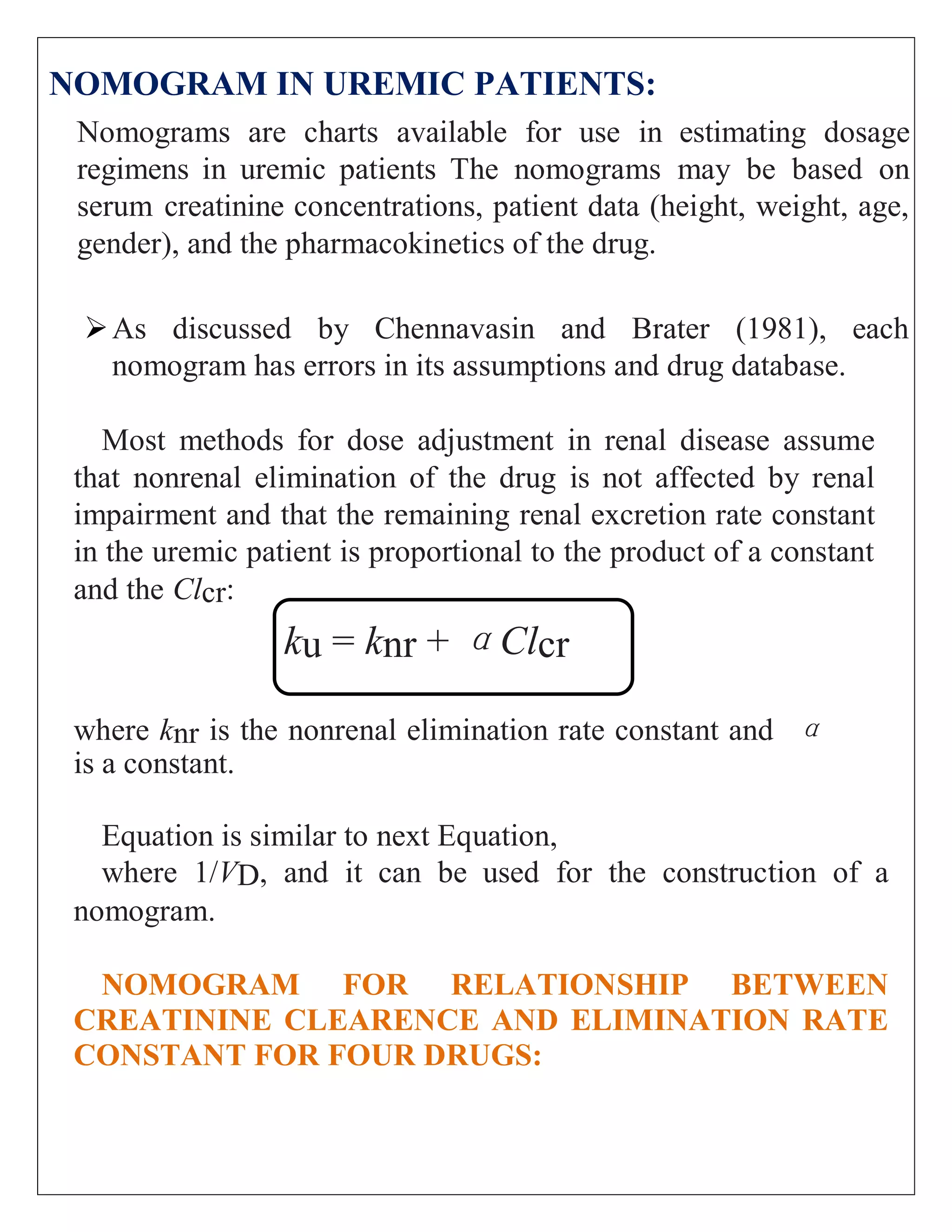
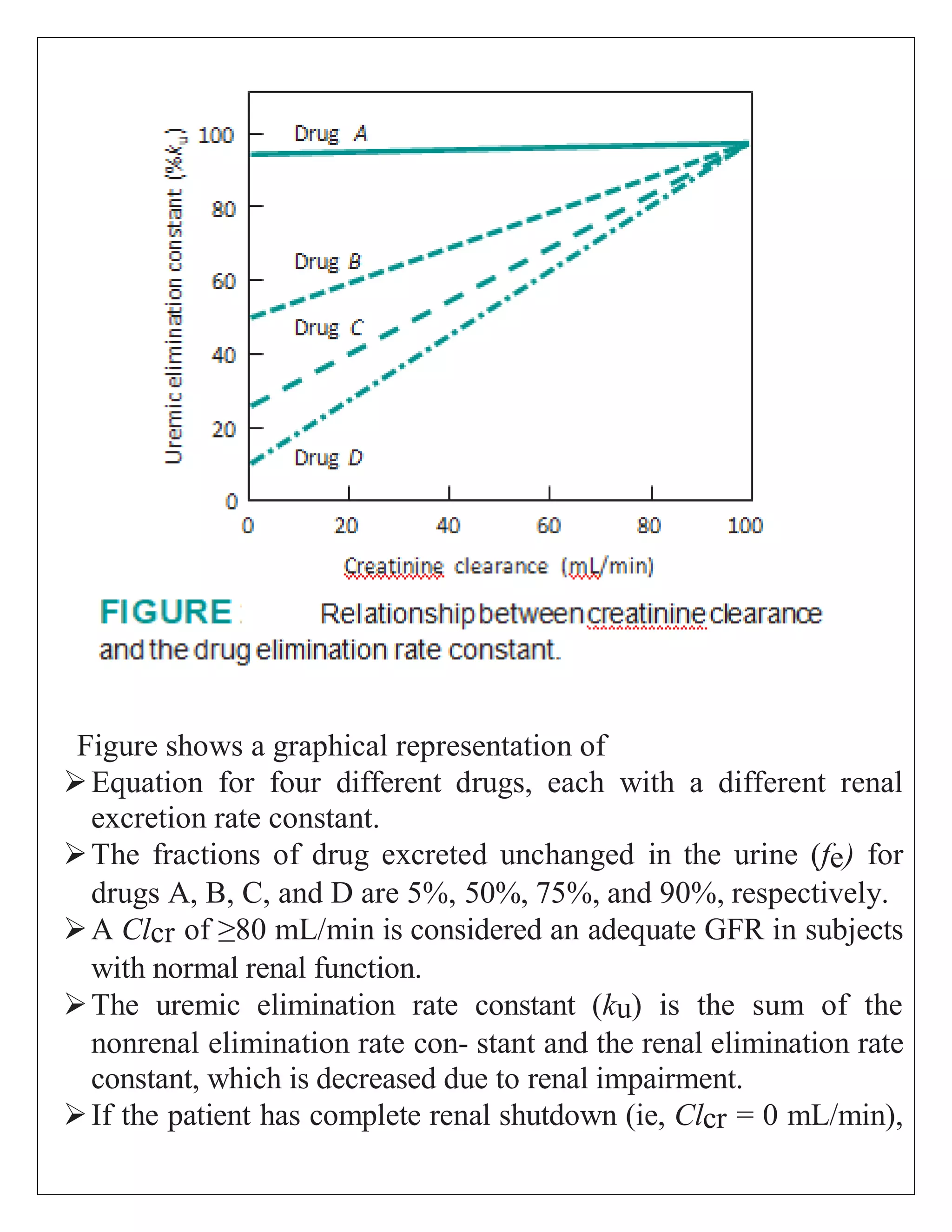
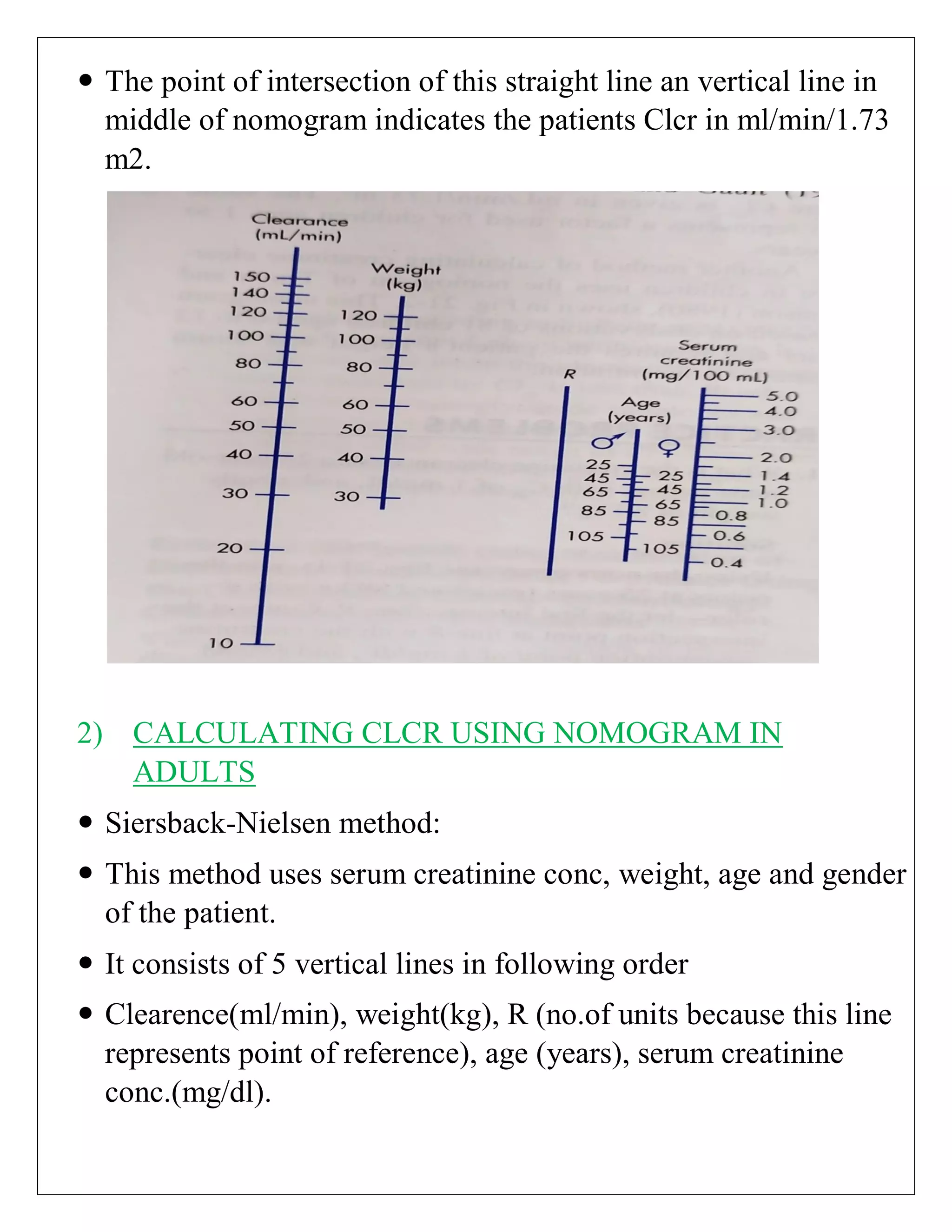
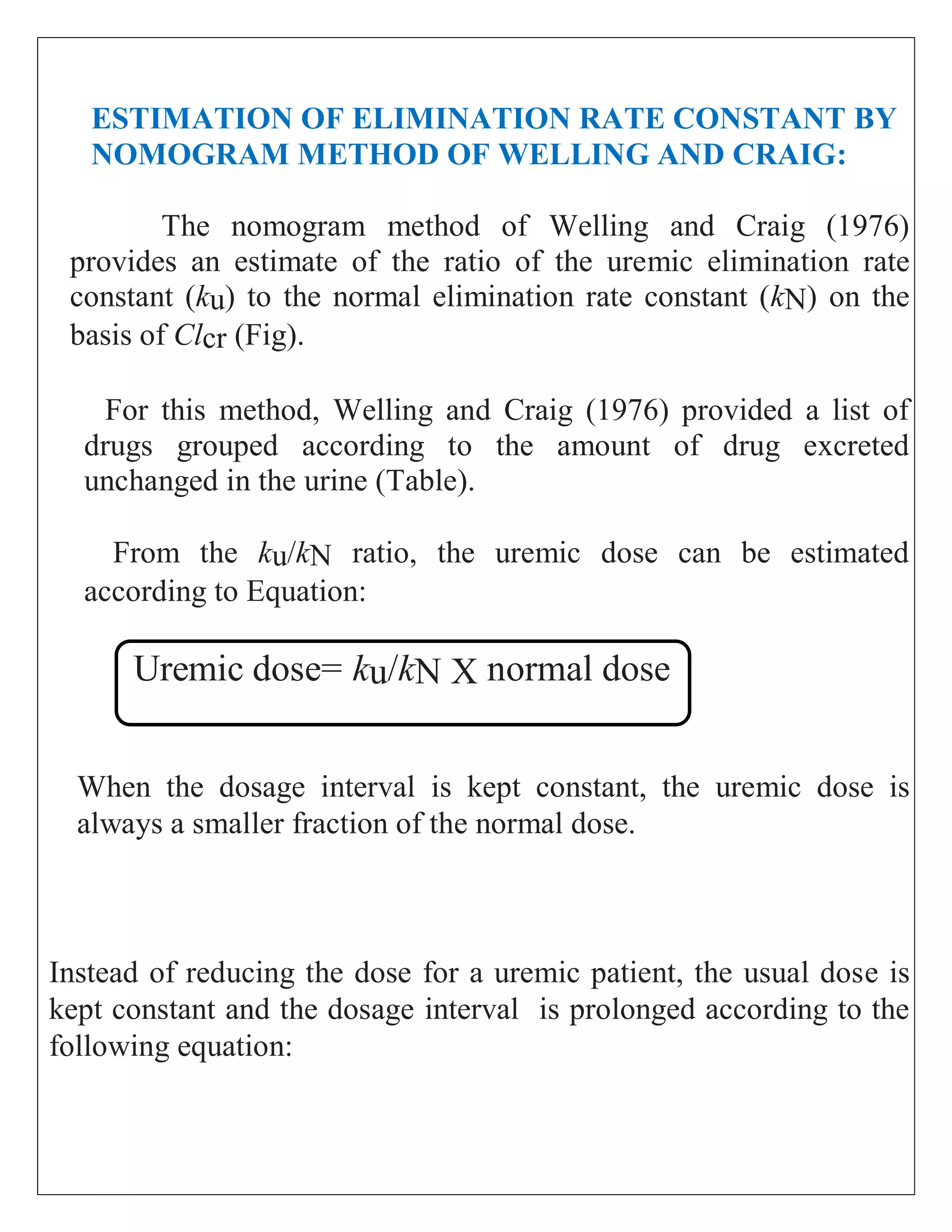
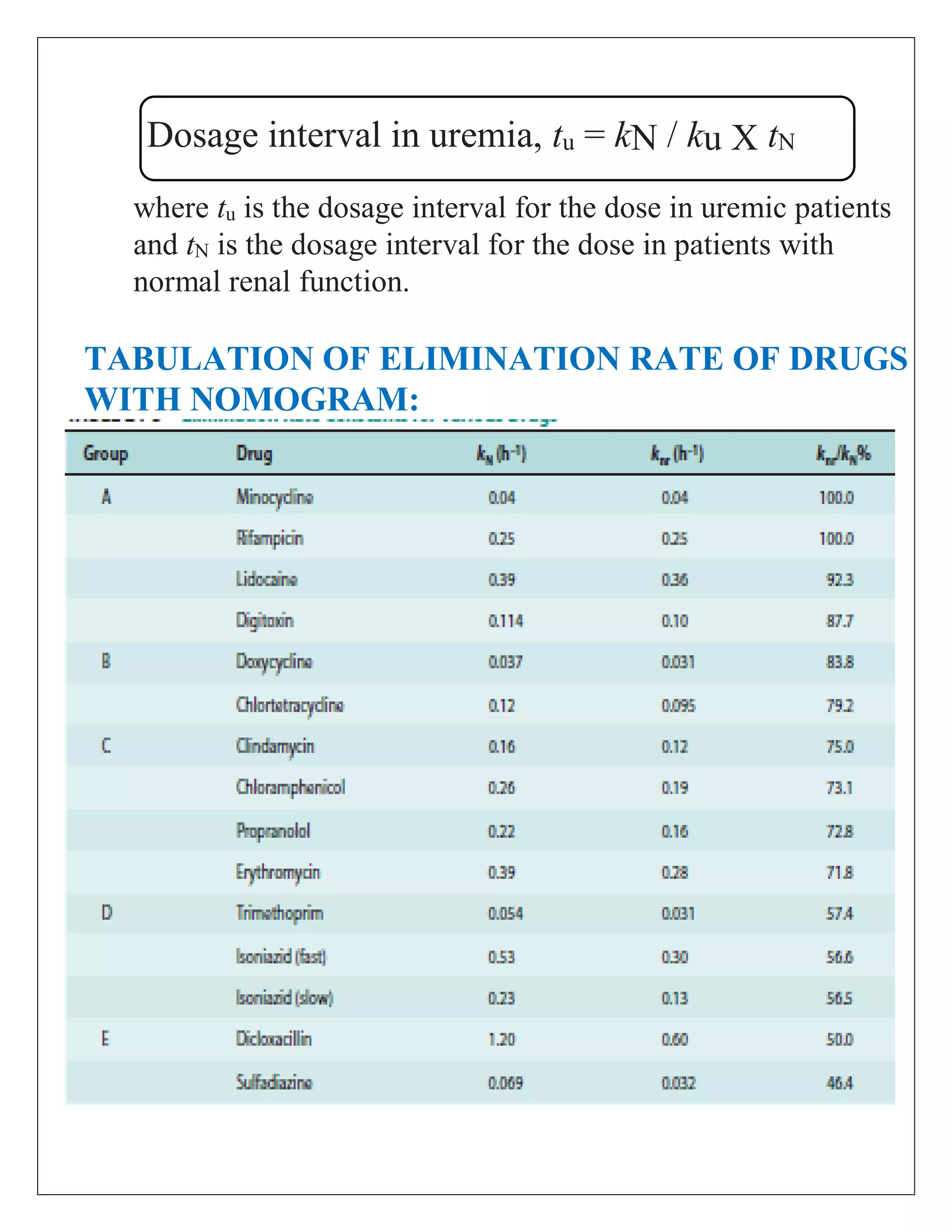
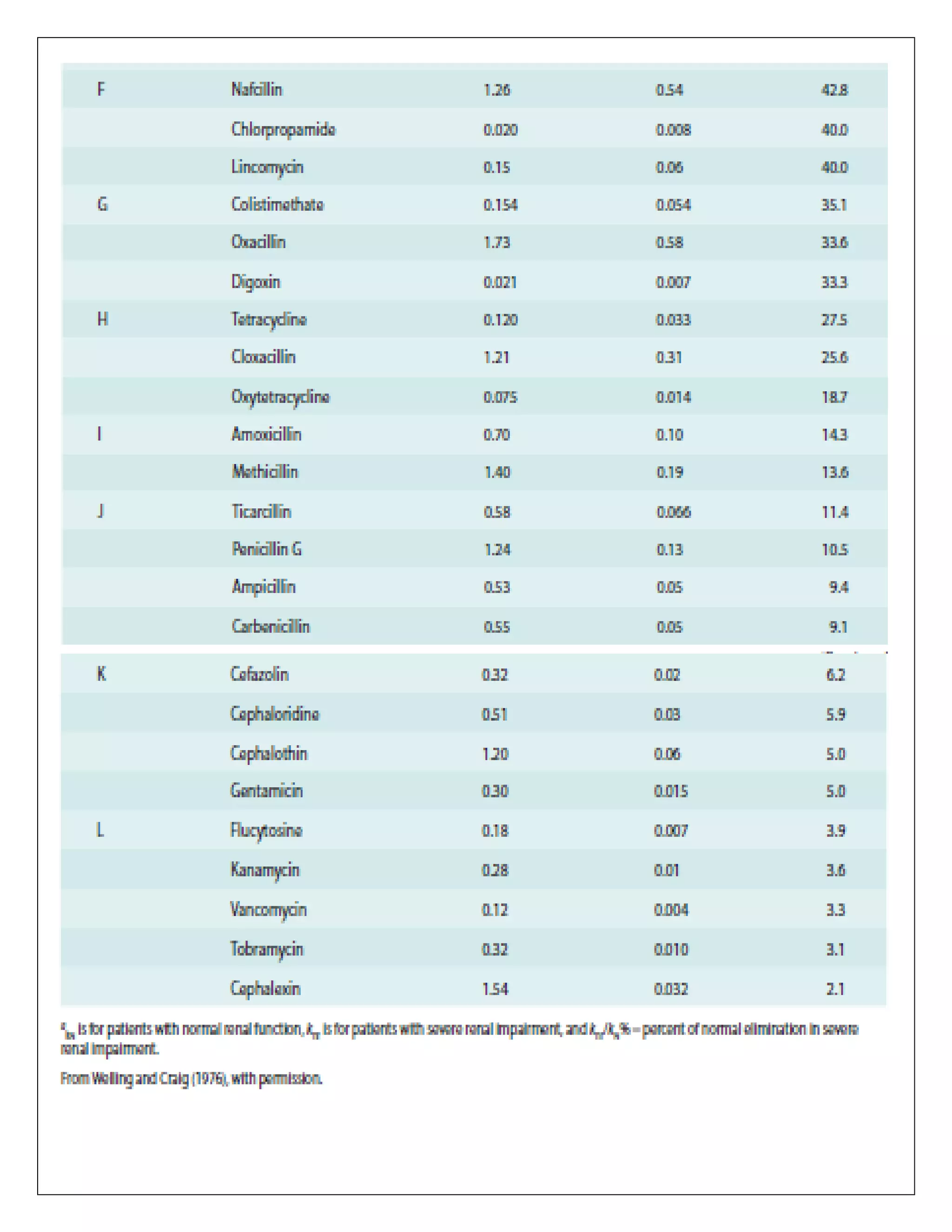
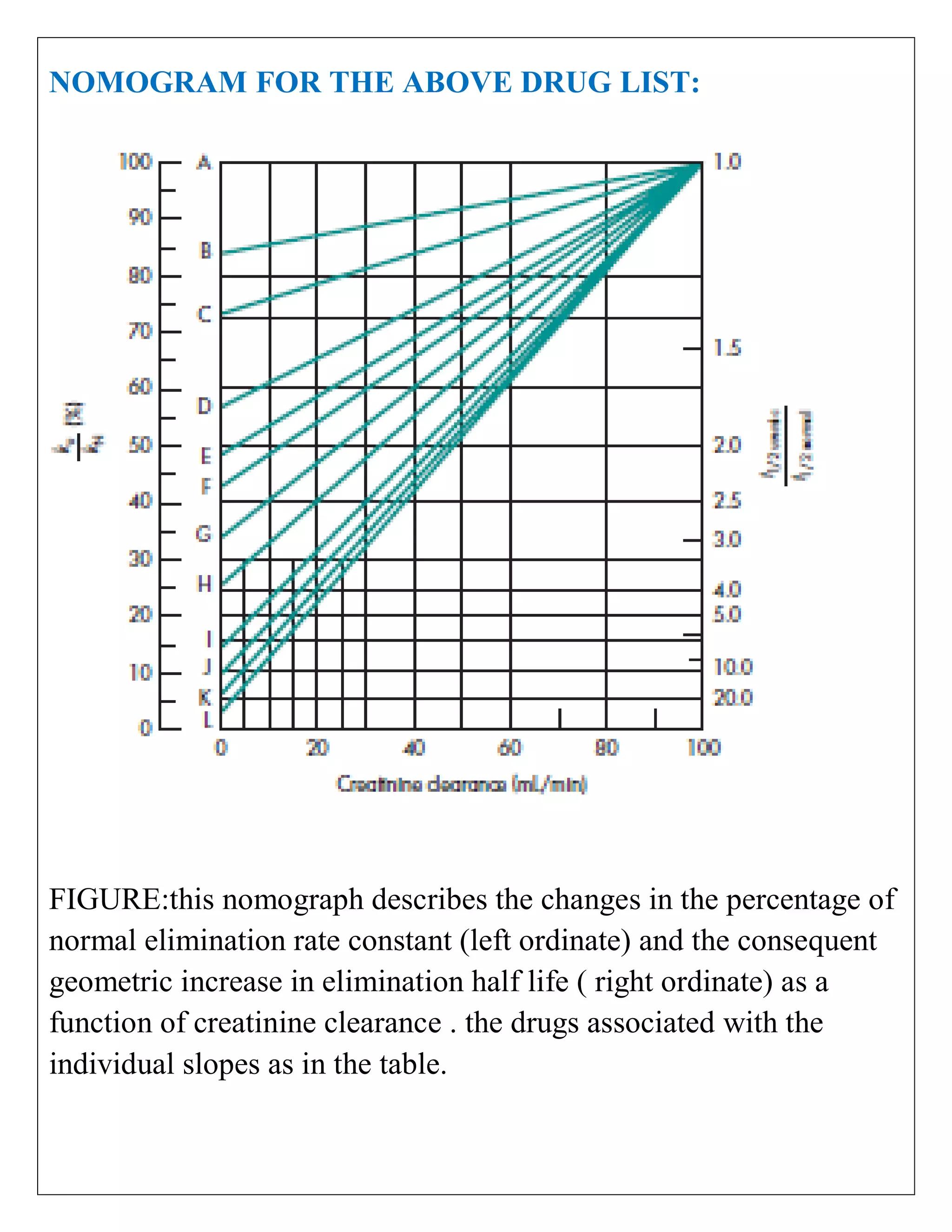
The document discusses the design of dosage regimens using pharmacokinetic parameters and highlights the importance of nomograms and tabulations for individualized patient dosing. It emphasizes the role of clinical judgment and the use of software for accurate calculations, as well as the considerations for specific populations such as pregnant women and those with renal impairment. Various methods, including nomograms for calculating creatinine clearance, are presented to aid in adjusting doses based on patient-specific factors.